
Image courtesy of Wegman's photo gallery
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Contents
One of the earlier talks in this series
used cooking as a metaphor for the gradual change in chemistry in the cosmos at large: elements such as hydrogen, helium and carbon were the ingredients of a cosmic recipe, and stellar interiors the ovens. I'll continue with that analogy in this brief presentation, which asks the question,
In ordinary life, the place to find the basic materials for a cake -- flour, sugar, butter, eggs -- is the supermarket.

Image courtesy of
Wegman's photo gallery
In the universe at large, the basic materials for, well, everything, were provided by an event we call the Big Bang. Let's take a closer look at it.
For our purposes, the Big Bang can be described simply as a time when the universe was
It has since cooled down, and it has since attenuated .... but it is still expanding. We have no idea what was going on at the very start -- our understanding of physics breaks down when the densities and temperatures become too high. I'll choose the time when the conditions reach this extreme and call it Curtains Up!. In the discussion below, I'll measure time starting with the raising of the curtains.
When the temperature was very high, above T = 1012 Kelvin, protons and neutrons existed in rough equibrium. With the amount of energy (and free particles) available at such high temperatures, protons could turn into neutrons and vice versa.
As time went on and the temperature dropped, the reactions converting neutrons to protons and vice versa fell out of balance. Going one way -- neutrons to protons -- was somewhat easier than going the other, and so protons became more and more common. About 3 seconds after the curtains went up, when the temperature dropped to around T = 1010 Kelvin, all these reactions fizzled to a halt. The mixture of protons and neutrons in this "primordial soup" was frozen at about 4.5 protons to every 1 neutron.

At a temperature of T = 1010 Kelvin, the soup was still too hot for atomic nuclei to form. If a proton and neutron (or proton and proton) happened to come together and fuse, they would immediately be broken up again by collisions with other particles or radiation.

Another 3 minutes or so passed while things were still too hot to fuse into nuclei. During this time, some neutrons decayed into protons; the half-life for this decay is about 885 seconds, so a small fraction of the neutrons turned into protons, making the soup a bit richer in protons: the ratio was now about 6 protons to every 1 neutron.
In real life, some recipes depend crucially on how long you keep the material on the heat.
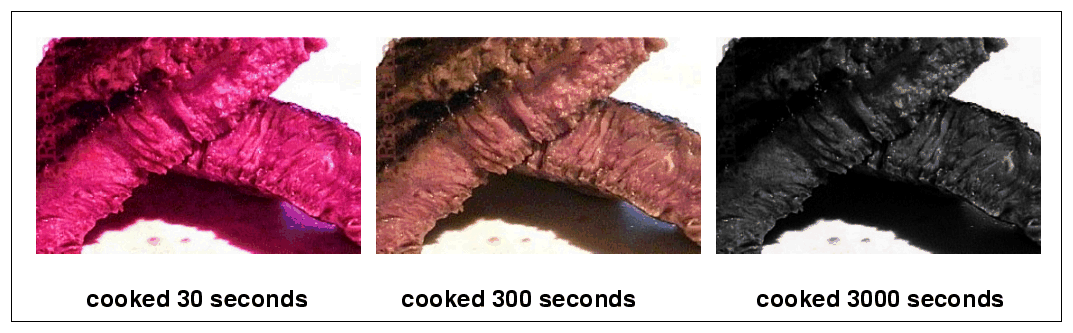
The same is true of the primordial soup. At temperatures below T = 109 Kelvin, the protons and neutrons can fuse to form simple atomic nuclei. The longer the simmering -- and the denser the material during the simmering period -- the more fusing takes place. The nuclei which can form during this "nucleosynthesis" are
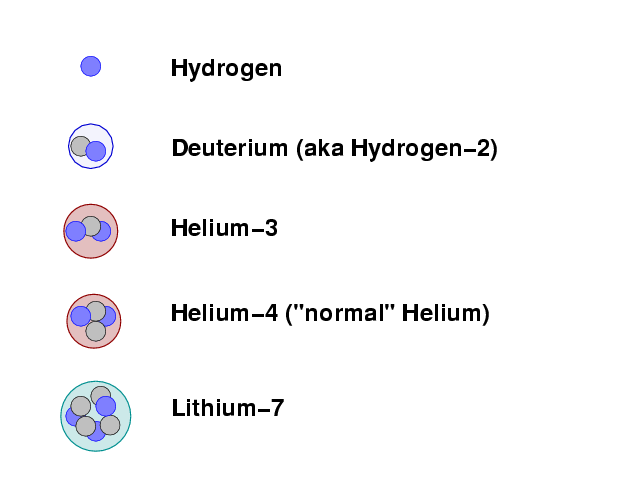
By far the most common reaction is for protons and light nuclei to combine to form Helium-4, the ordinary form of helium. The denser the soup, and the longer it simmers within the appropriate range of temperature, the more hydrogen is turned into helium. The relative amounts of the other products -- deuterium, helium-3 and lithium-7 -- also depends on density and duration.
The energies of particles during this "simmering" phase of the universe are between 0.1 MeV and 1.0 MeV, which can easily be reproduced in terrestrial laboratories. Physicists have measured very accurately the rates at which all the various reactions between these particles and nuclei take place, and can make confident predictions for the relative amounts of each product after any particular duration of time. One can put all the information into a single picture:
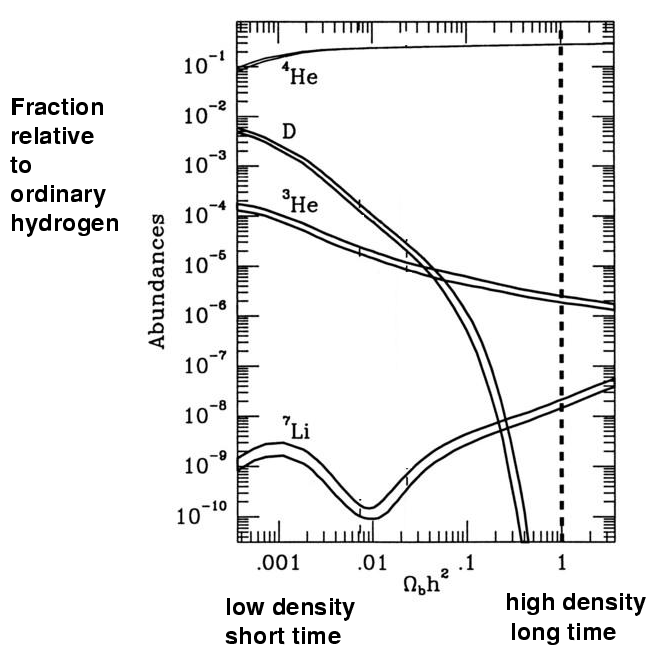
To read this graph, pick some particular combination of density and duration (denoted by the symbols Ωbh2) along the bottom axis; then, draw a vertical line at that position. Where the line crosses each element's pathway is the predicted relative abundance of that element.
For example, in a low-density universe, we ought to find
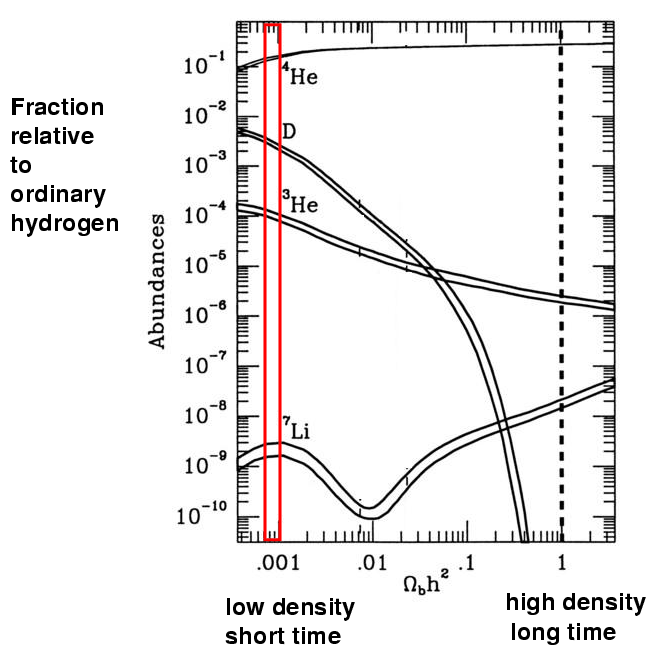
If this whole story about a hot, dense period of the universe is true, then the current mixture of these light elements ought to fall into one of the predicted combinations. If it does, we can conclude not only
but also
Astronomers have spent many years observing stars, galaxies, and nebulae very carefully with big telescopes and spectrographs. As described in the first article in this series, spectra of objects can tell us their chemical composition. For example, below is a spectrum in the ultraviolet of a distant star in our Milky Way Galaxy. Gas in clouds between us and the star absorbs some of the starlight. The strength of the normal hydrogen absorption lines and the neighboring, weaker deuterium lines provides an estimate of the relative amounts of hydrogen and deuterium in the gas.
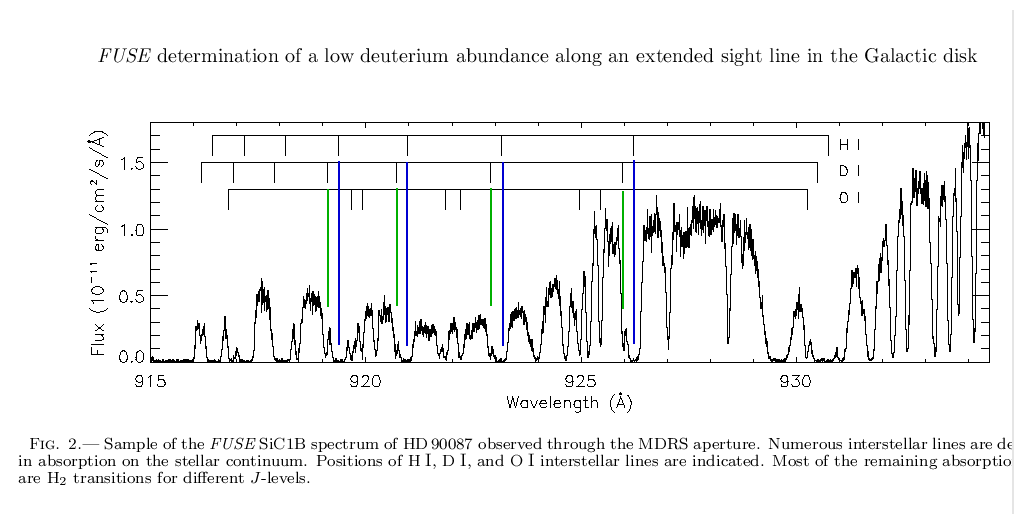
From a recent paper by Hebrard et al.,
Astrophysical Journal, Vol 635, pp. 1136-1150.
So, here's a recent summary of the observations, from a paper by Richard Cyburt.
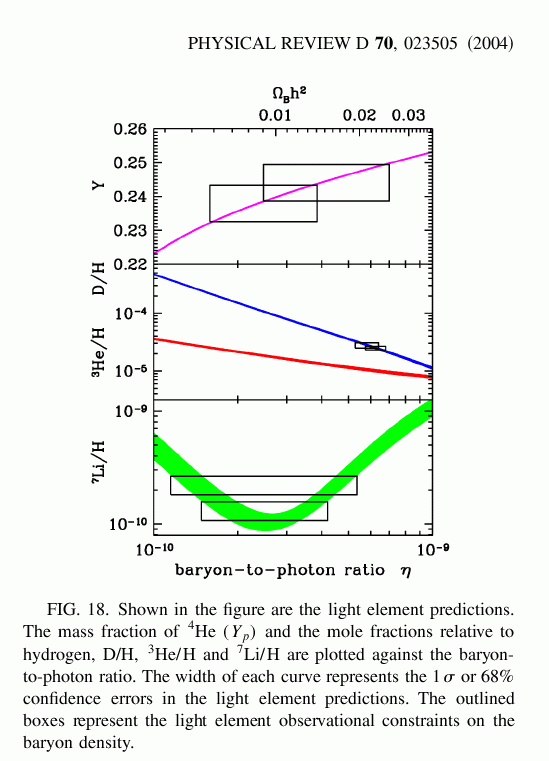
What this tells us is
and
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.