 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Contents
People have long known that the stars are far, far away; in the nineteeth century, astronomers finally measured the distances to a few nearby stars with reasonable accuracy. The results were so large -- thousand of trillions of miles -- that most people figured we'd never be able to visit them or learn much about them. After all, we can't go to a star, grab a sample, and bring it back to earth; all we can do is look at light from the star. In fact, at least one prominent philosopher and scientist went on the record as saying that we'd never be able to figure out their compositions.
Of all objects, the planets are those which appear to us under the least varied aspect. We see how we may determine their forms, their distances, their bulk, and their motions, but we can never known anything of their chemical or mineralogical structure; and, much less, that of organized beings living on their surface ...
(Comte refers to the planets in the quotation above; he believes that we can learn even less about the stars)
Within thirty years, however, scientists were indeed starting to investigate the chemical composition of the Sun, planets, and some bright stars. How did they do it?

No, they didn't travel to the Sun.
Instead, they took advantage of the information carried by the photons which did travel from the Sun to the Earth.
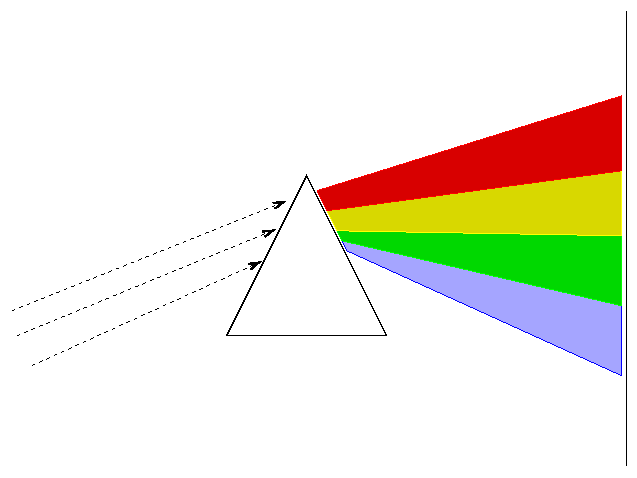
A spectrograph takes light from a source and separates it by wavelength, so that the red light goes in one direction, the yellow light in another direction, the blue light in another direction, and so forth. One type of spectrograph depends on a prism to disperse the light:
Astronomers often place a slit over the focal plane of the telescope, centered on the object of interest.
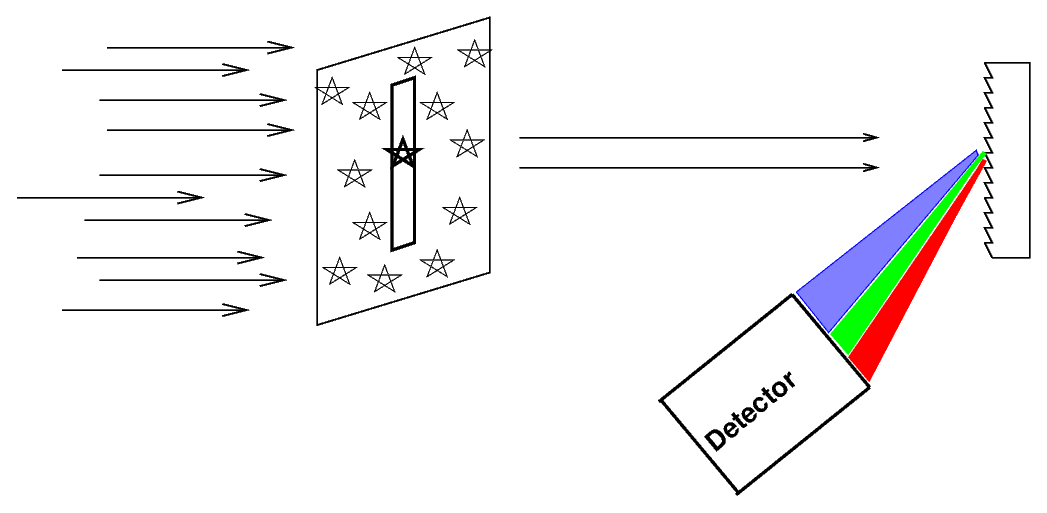
Only light which passes through this slit will strike the grating (or prism), giving the spectrum a characteristic shape: vertical lines on a long, horizontal canvas.
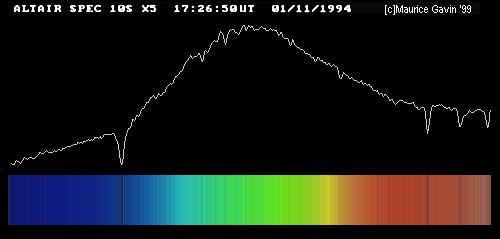
Image by Maurice Gavin, from the wpo-amateur spectroscopy web site.
When we pass light from a source through a spectrgraph, we usually see one of three basic types of spectrum, depending on the nature of the source. German astronomer Gustav Kirchoff, working in the 1850s, figured out the reason for these different types of spectra. He explained the three basic types of spectra as coming from three different situations:

![]()
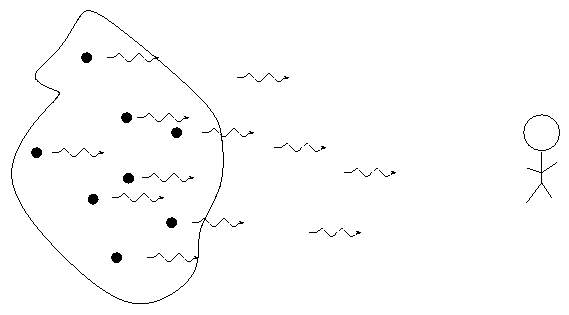
![]()
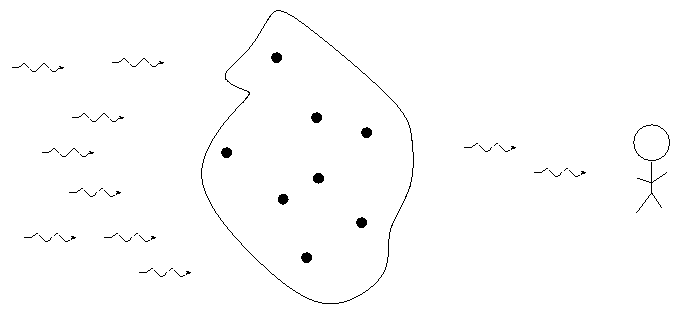
![]()
Each element generates its own unique set of wavelengths of emission or absorption.
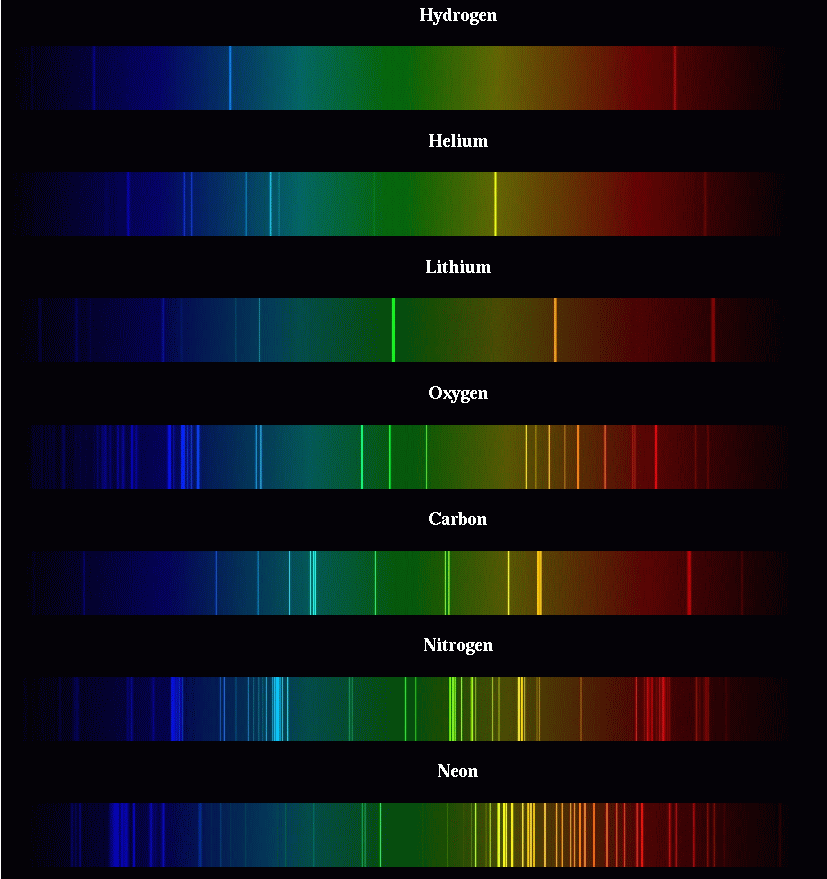
We can use these patterns like fingerprints to identify the material which is emitting or absorbing light.
So, astronomers took spectra of the Sun (and stars), and tried to identify as many lines as they could. This isn't easy, since there are a LOT of absorption lines in the visible portion of the Sun's spectrum:
However, as the years went by, scientists were able to track down the identity of some of the strongest lines. They found that the most prominent lines in the solar spectrum are due to the elements
So, the conclusion was that these were the most abundant elements in the Sun. Does that make sense? Sure! After all, compare that list to these lists of the most common elements in the Earth's crust, and in the entire body of the Earth:
In the Earth's crust In the entire Earth
Element percentage abundance Element percentage abundance
by weight by number by weight by number
-------------------------------- ----------------------------------
oxygen 46.6 2.91 iron 34.6 0.618
silicon 27.7 0.99 oxygen 29.5 1.84
aluminum 8.1 0.30 silicon 15.2 0.543
iron 5.0 0.089 magnesium 12.7 0.529
calcium 3.6 0.098 nickel 2.4 0.041
sodium 2.8 0.12 sulfer 1.9 0.059
Simple, easy, and makes sense: the Sun's chemical composition is pretty similar to the Earth. Everything hung together.
Unfortunately, it was WRONG.
In 1925, a young graduate student named Cecilia Payne finished her Ph.D. dissertation, Stellar Atmospheres: A contribution to the observational study of high temperature in the reversing layer of stars. She realized that the situation in the outer atmosphere of the Sun and other stars was not so simple after all. The basic picture -- which was correct -- went something like this:
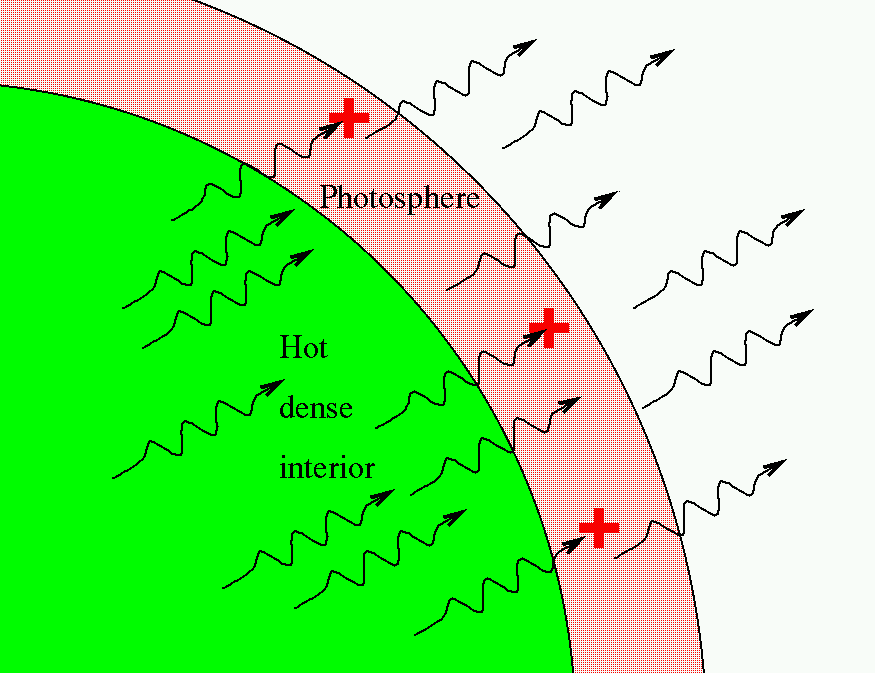
If the photosphere of a star contained atoms of a particular element, then one would expect to see absorption lines at the wavelengths associated with that element. At the risk of simplifying things a bit (well, a lot), the understanding of the time was sort of like this:
strong absorption line ----> lots of that element weak absorption line ----> little of that element no absorption line ----> none of that element
Payne thought carefully about some of the most recent results from atomic physics, which was in the middle of its quantum revolution. She realized that the strength of an absorption line did depend on the abundance of some element, but that there were other factors which were just as important -- or even more important:

From Chapter XIII of Payne's dissertation.
In order to explain Payne's work in detail, one must delve into the quantum energy states of atoms, and their interaction with packets of light. Hmmm....
Perhaps it would be better to work with an analogy. Consider the quantum energy states of Fred, an ordinary guy, and his interaction with cups of coffee.
First, consider what happens when a cup of coffee encounters Fred in his state of lowest energy:
Result: no absorption. The coffee escapes and is free to continue its journey.
Now, suppose we give Fred a little bit of energy -- so that he is awake, but sitting at his desk and doing paperwork. What happens when coffee approaches him now?
Result: absorption. The coffee disappears.
If we give Fred lots of energy, so that he runs a marathon, what will happen when he encounters the cup of coffee?
Result: no absorption. The coffee escapes and is free to continue its journey.
In the same way, Payne showed that atoms of a particular element in the solar atmosphere would only absorb passing photons of light if the atoms were in exactly the right atomic energy state. This meant that just because one element -- say, iron -- creates the strongest absorption lines, it may not necessarily be the most abundant type of atom. There might be (to continue our analogy) millions of "sleeping" atoms of some other sort which are present, but just don't absorb the light.
So, with our current understanding of atomic physics and spectroscopy, and with our current telescopes and spectrographs, we believe that the chemical composition of the Sun is as follows:
relative rough
element 12 + log(N/Nh) abundance percentage
by number by number
----------------------------------------------------------------
H 12 1.0 92 percent
He 10.93 0.085 7.8
O 8.66 0.000 457 0.041
C 8.39 0.000 245 0.022
Ne 7.84 0.000 069 0.0064
N 7.78 0.000 060 0.0055
In other words, a lot of hydrogen and and a little helium, with tiny bits of heavier elements.
The Sun does NOT contain, on average, the same material as the Earth. Neither do stars, or clouds of interstellar gas, or the Milky Way as a whole.
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.