 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Contents
I'll try to be brief.
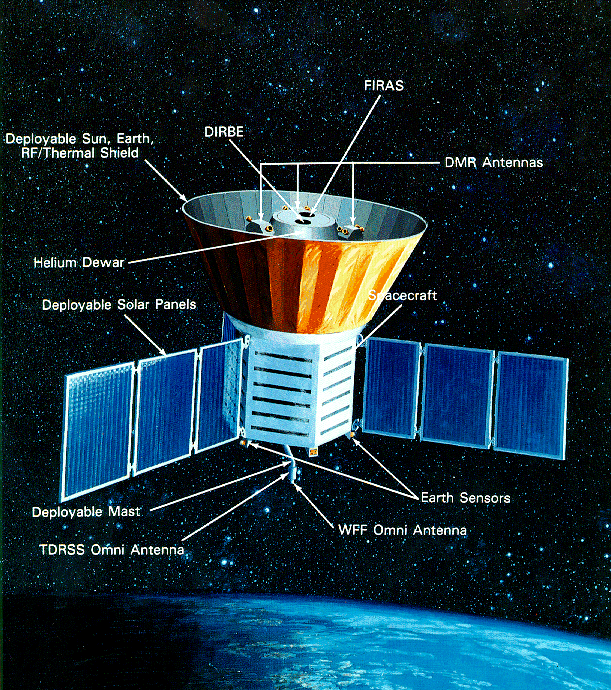
The 2006 Nobel Prize in Physics was awarded to two men: John Mather and George Smoot. Both worked on the COsmic Background Explorer (COBE) satellite , which was designed to make very precise measurements of radiation produced in the early universe.

Each man was the architect of one of COBE's instruments.
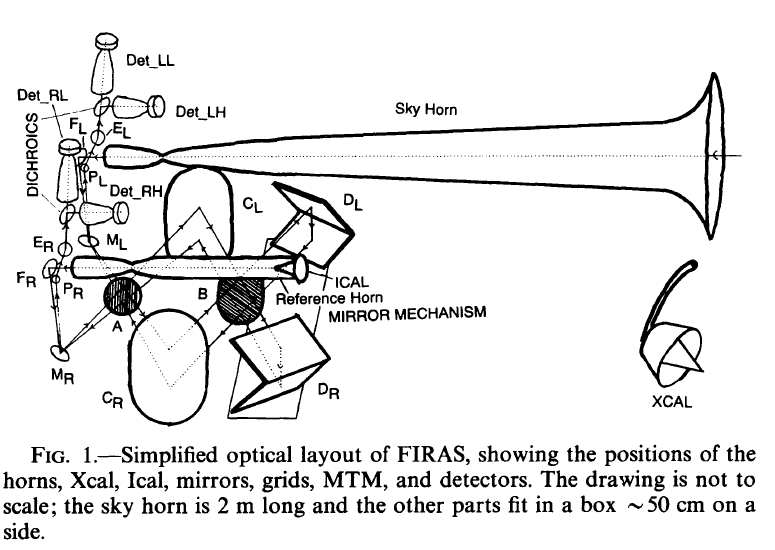
Image from
Mather et al., ApJ 420, 457 (1994)
There was a prediction that the cosmic background should glow in the same manner as a hot block of metal:

This image courtesy of
Kevin Baird
Physicists call this sort of glow blackbody radiation. If theories were correct, and the microwave background was the remnant of radiation emitted by hot, dense gas long ago, then the spectrum of this radiation should have a very specific shape. The figure below compares the theory (blue line) with the measurements (34 points in blue).

The differences between the theoretical blackbody spectrum and the measurements are pretty darn small.
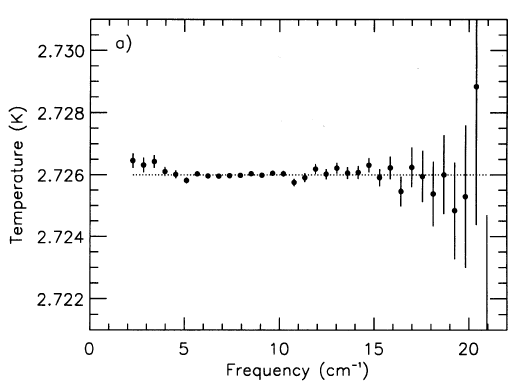
Image from
Mather et al., ApJ 420, 439 (1994)
Image taken from
Smoot et al., ApJ 360, 685 (1990)
The DMR found that the microwave intensity was almost uniform:
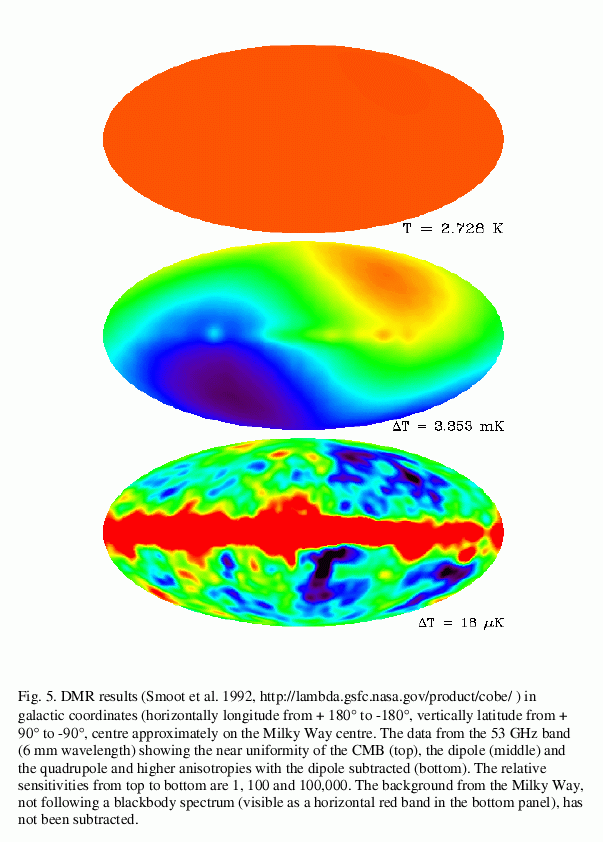
However, once one removed the dipole, and then the emission from gas in the Milky Way Galaxy (as well as one could), what was left were very small variations in little patches: some a bit brighter, some a bit fainter.
The sizes of these fluctuations can tell cosmologists about the clumpiness of the gas in the very early universe; that clumpiness evolved over a very long time into the lumpy distribution of matter in the current universe.
We saw in last month's talk that the Sun is mostly hydrogen. That turns out to be true for the vast majority of stars. In fact, we believe that the primordial material from which the very first stars formed was mostly hydrogent, too (that very early universe will be the topic of the third talk in this series).
So, if the universe was once mostly hydrogen, with a bit of helium, whence came all the heavier elements?
Table courtesy of the
Visual Periodic Table page.
The answer is: light elements were baked into heavier elements inside stellar ovens. Scientists may call the process "stellar nucleosynthesis", but it's basically cooking. Let's take look at several of the most common recipes in the universe.
We'll start with a very simple recipe: stewing hydrogen into helium. The basic idea is to combine 4 Hydrogen nuclei (protons) so that they form a single Helium nucleus (2 protons and 2 neutrons):
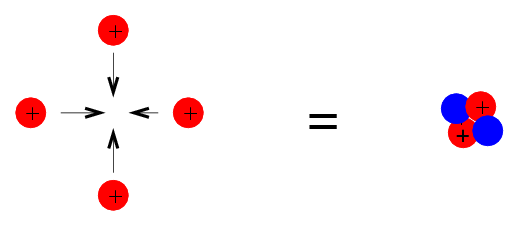
There's one difficulty with this process -- what is it?
Right. The problem is that protons are all positively charged particles, and so repel each other electrically. In addition, the probability that 4 protons to run into each other simultaneously is very small -- so this won't happen very often at all.
Nevertheless, a clever chef can still make a nice helium stew; it just takes a little special processing. There are two possibilities:

This image courtesy of
mrjojo .
If we wait long enough, this sequence of reactions will occasionally take place:
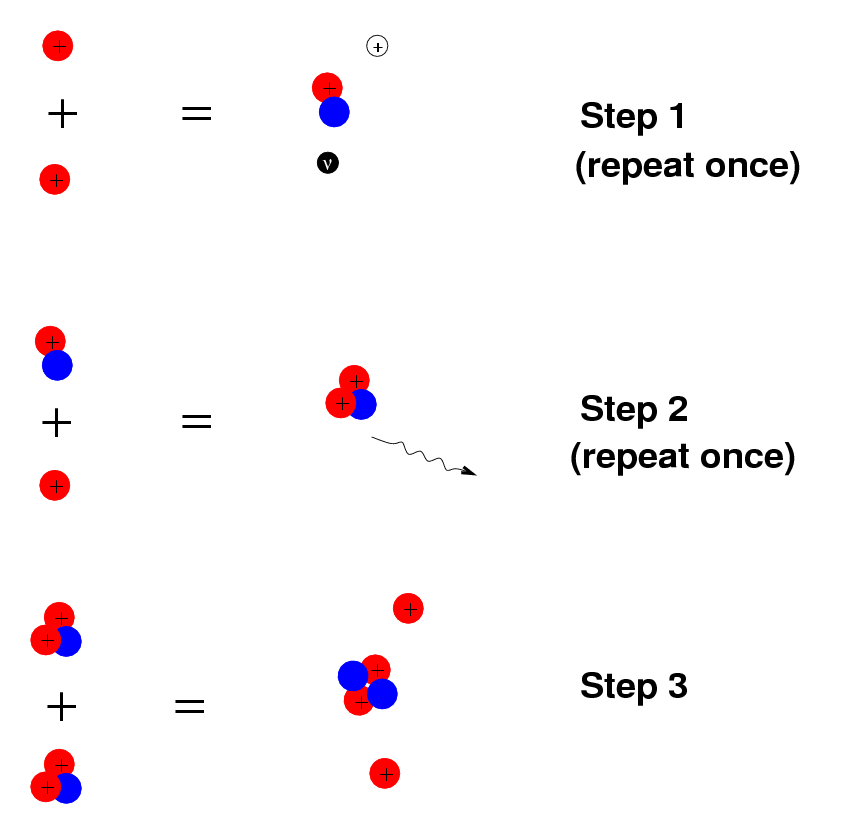
Starting with 6 protons, we end up with 2 leftover protons plus one helium nucleus; oh, and some energy and 2 neutrinos are produced, too.
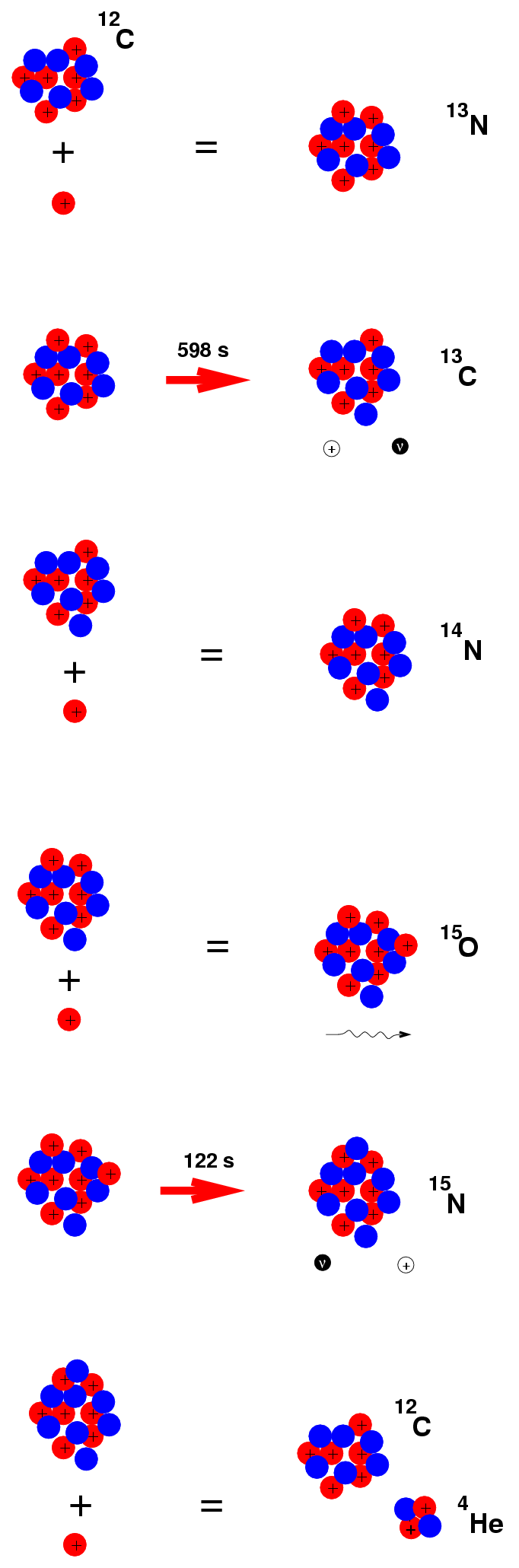
We started with 4 protons and 1 Carbon-12 nucleus; we end up with 1 Helium nucleus and 1 Carbon-12 nucleus. The Carbon-12 acts as a catalyst: it allows the long sequence to begin, but isn't consumed. Because the reactions involve not only Carbon but Nitrogen and Oxygen, this recipe is called the CNO cycle.
Okay, so far, our cookbook allows us to start with Hydrogen and create Helium. But that leaves over 90 tasty elements as yet unmade.
Table courtesy of the
Visual Periodic Table page.
Suppose that we try to use the same techniques by adding protons to helium -- that should make Lithium, right? And it does. Some of the Lithium will combine with protons to make Beryllium, too. So our pot will become a blend of Hydrogen, Helium, Lithium and Beryllium.
Unfortunately, at this point, we reach a sticking point. At the temperatures and pressures inside our Sun, our stew will remain in this state. It won't brew any heavier elements. The problem lies with the nature of Beryllium and Boron nuclei: the isotopes of these elements which are the stepping stones to heavier elements are either radioactively unstable, so that they break up as soon as they are formed, or are so weakly bound that the jostling by other particles destroys them. This is as far as things can go inside our own Sun, at the moment.
In order to progress to really interesting elements, we need to change the conditions: pour the stew into a pressure cooker and increase its density, pressure and temperature. Our Sun will become this sort of pressure cooker near the end of its life; more massive stars have these cookers in their kitchens from the start.

Inside our pressure cooker, the triple-alpha process may take place, in two steps:
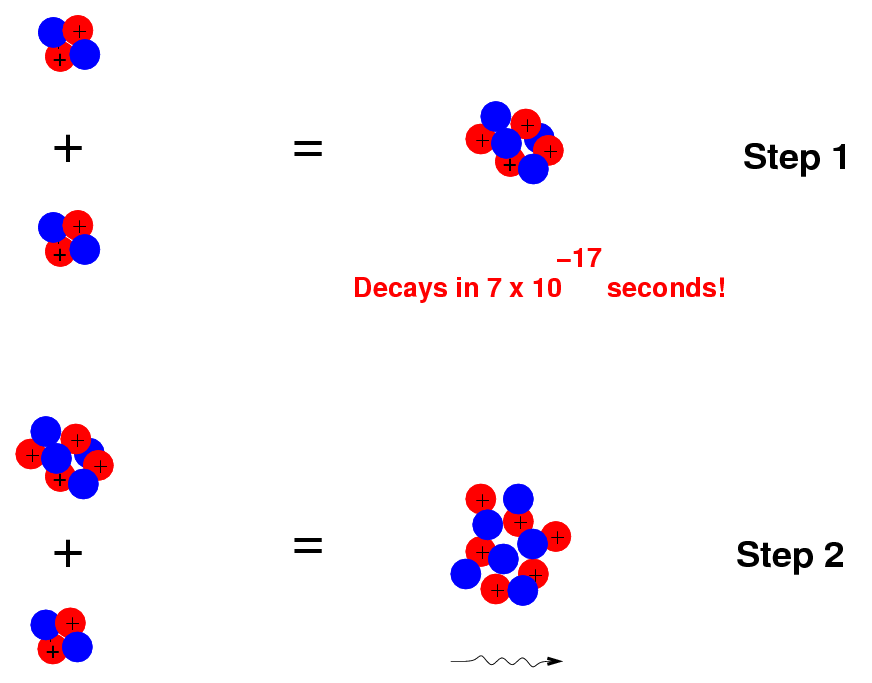
The net result is to turn 3 Helium nuclei into a single Carbon nucleus. Once our stew has some Carbon, it can slowly add protons to the Carbon to build up heavier elements: Nitrogen, Oxygen and so forth.
It turns out that the pressure-cooker technique allows one to create all the elements up to a point ...
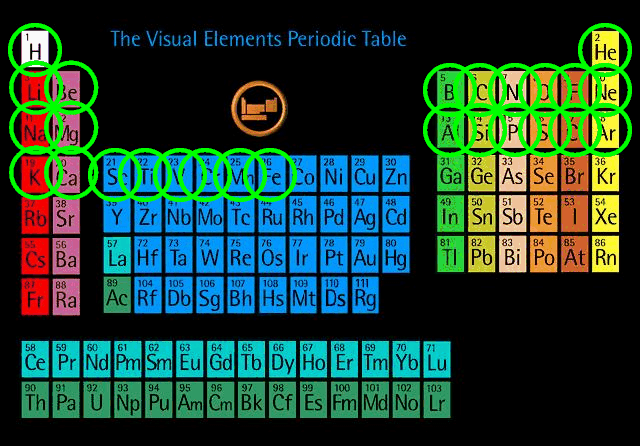
Table courtesy of the
Visual Periodic Table page.
Iron is a special element: its nucleus is very stable. If one tries to add a proton or two to it, to create a heavier element, the reaction sucks a bit of energy out of the pot. All the other reactions we've discussed so far actually release energy, keeping our stew bubbling nicely. But if you try to build elements heavier than iron, your pot will cool off, and the reactions will cease.
The only way to form elements much heavier than iron is add an overwhelming amount of energy to the stew very, very quickly.

Castle-Bravo image courtesy of
ZVis' nuclear gallery
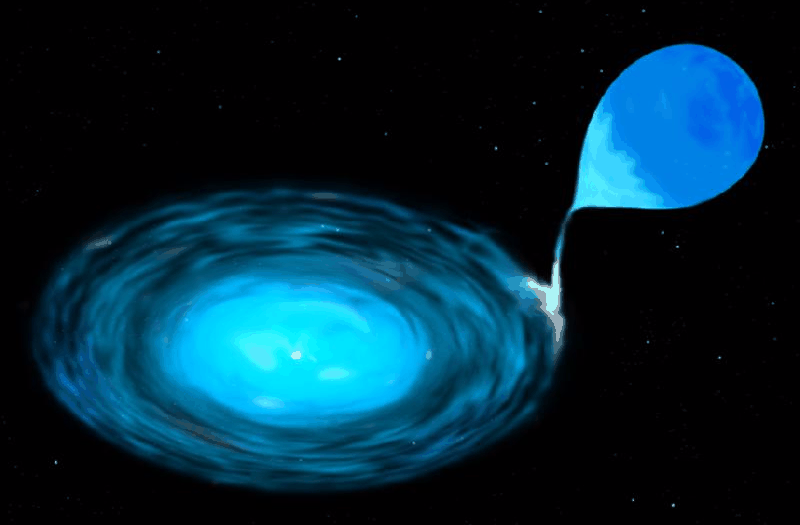
There only situations which can provide a large enough flux of free energy within a super-dense environment are supernovae, stars which explode. There are two varieties,
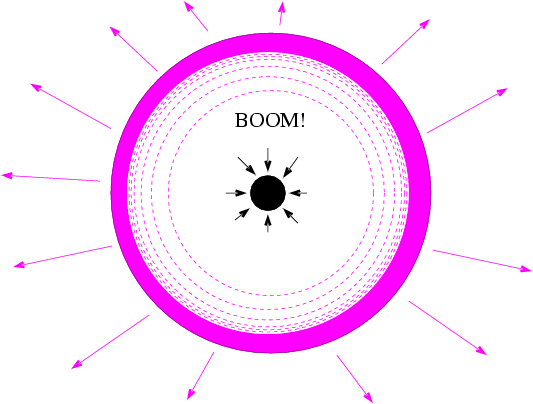
Under these extreme conditions -- which last for no more than a few seconds -- nuclei of iron and lighter elements are forced together to form all the heavier elements in the periodic table.
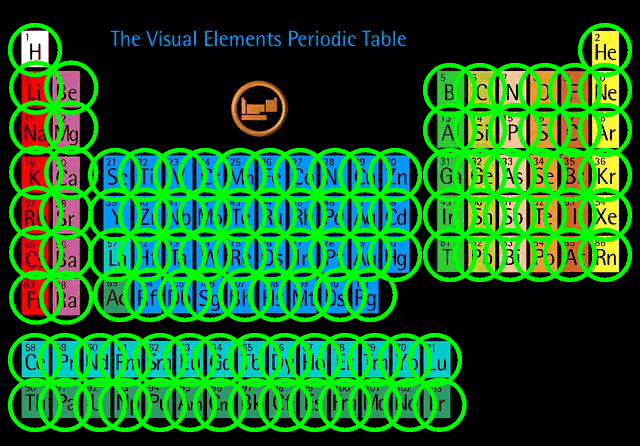
All the heavy elements in this room -- such as the gold, silver and platinum in the jewelry we wear -- was formed within the core of an exploding star ...
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.