
Image of aurora over Rochester courtesy of Sonikku_a
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.

Image of aurora over Rochester courtesy of
Sonikku_a
Contents

Image of aurora over Webster Park in 2024 courtesy of
meloncap78

Image of aurora over Rochester in 2024 courtesy of
UnicornMafia69

Image of aurora over Irondequoit in 2024 courtesy of
bombers00
Finally, here's a slightly different view of an aurora over our skies.
Q: Can you find Rochester? (click to play the movie)
Movie of aurora over North America courtesy of
NASA's collection of ISS aurora movies
(Here's a hint if you're still looking for Rochester.)
Suppose we wish to cook up our own light show in the night sky. What items do we need? How must we prepare them? How will our choices affect the final display of colors?
Let me try to provide a very basic explanation. I'll simplify as much as I can, but try to maintain some connection to physical reality. As part of this explanation, we'll carry out a little demonstration with some materials that I have brought.
Most of the gas in the Earth's atmosphere -- and almost all all of the gas in this room right now -- is molecular: two or three atoms join together to form a larger entity, such as N2 (two nitrogen atoms forming an nitrogen molecule) or CO2 (one carbon and two oxygen atoms forming a molecule of carbon dioxide). Our bodies require molecular oxygen to run our respiratory systems properly.
The trouble is that when atoms combine into molecules, the great majority of their interactions -- as they collide with each other, bump into walls, absorb and emit light -- involve changes in energy which are relatively low. That means that the photons emitted by gas molecules tend to lie in the INFRARED portion of the spectrum ... which is invisible to human eyes.
On the other hand, isolated atoms often emit light with somewhat higher energies, in the VISIBLE portion of the spectrum. So, if we want to see something happening in the sky, it helps to have gas made of isolated atoms.
Consider, for example, the simplest atom of all: hydrogen. It consists of a single proton at its center, around which a single electron orbits. It turns out that the electron can only circle the proton at a certain set of distances, which correspond to a certain set of energies.
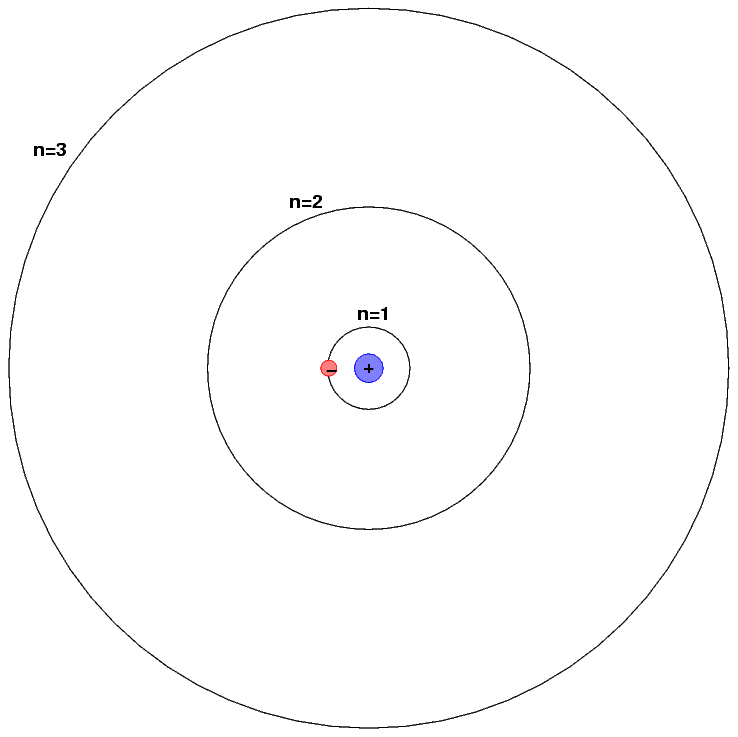
Because circles are awkward to draw, scientists often draw the energy levels of a hydrogen atom (corresponding to the different circular orbits) as a series of flat lines; it's just more convenient. Each orbit has a certain energy associated with it. In atoms with lots of energy, electrons will be in high levels, such as n=5 or n=10, while in atoms with very little energy, the electrons will occupy a low level, such as n=1.
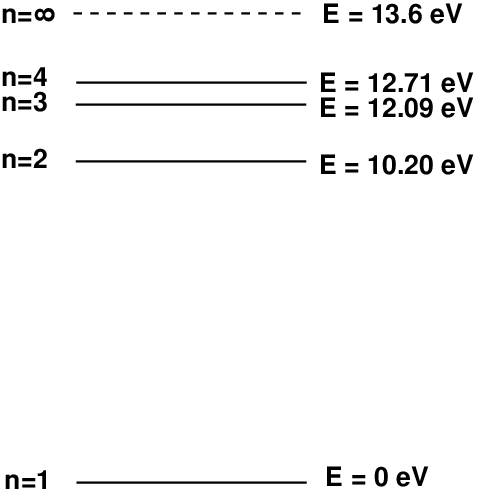
Okay, here comes the important part: an atom which has a lot of energy can release some of its energy as a photon by "jumping" from one energy level to another. The energy of that photon -- which determines its wavelength and color -- is simply the difference between the atom's original energy and its final energy.
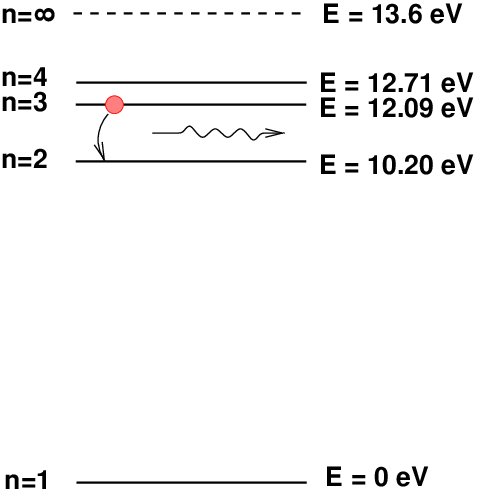
In this particular example, a hydrogen atom will emit a photon with an energy of 1.89 electron-Volts (eV), which corresponds to a wavelength of 656 nanometers (nm), or a color of deep red. Many nebulae in the sky are rich in atomic hydrogen gas, and so many pictures show this rich, deep red color.

Image of M8 courtesy of
Ignacio Diaz Bobillo and
Astronomy Picture of the Day
Now, each element -- hydrogen, helium, oxygen, nitrogen, etc. -- has its own unique set of atomic energy levels. That means that each type of gas will emit its own unique set of wavelengths. Scientsts can use instruments called "spectrographs" to break light into a rainbow (or "spectrum"), which allows us to identify the specific wavelengths emitted by some source of light. If we can identify the wavelengths, we can identify the elements!
One way to break up light into a spectrum is to shine it through a prism of glass, like this:
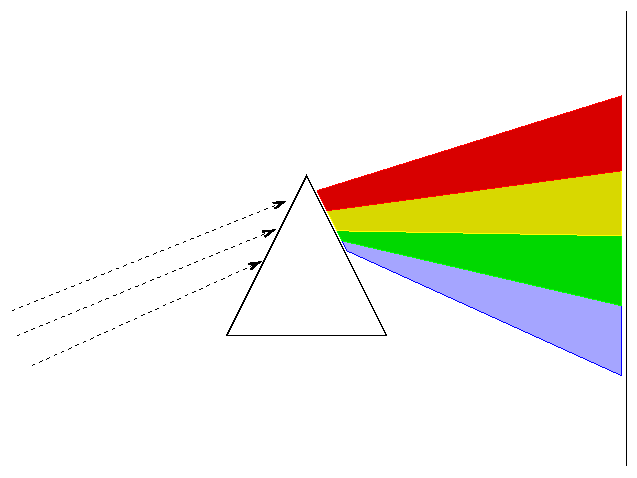
It would be a bit awkward to hand out many little pieces of glass to you in the audience, so I've chosen a different tool for breaking light into spectrum. DIFFRACTION GRATINGS use a thin plastic film with many parallel grooves to send light of different wavelengths into different directions.
As a little exercise, let's see if you can use the colors of light emitted by different tubes of gas to identify the element in each one. Here's a visual guide to the spectra of some common elements.

So, what can we use to excite atoms into high energy states?
One possibility, of course, is ordinary sunlight. The photons we see as we walk around on Earth every day are plentiful, and they do carry energy. Some of them do collide with atoms of gas high in the Earth's atmosphere ... but there are a couple of problems.
The problem is that our Sun is a relatively cool star -- the temperature of its outer atmosphere is only about 5800 Kelvin = 10,000 Fahrenheit. That means that most of the photons emitted by the Sun are in the green-yellow-orange-red portion of the visible spectrum (or, at even lower energies, in the invisible infrared region). These photons can't impart enough energy to atoms in the Earth's atmosphere to raise them up out of the low ground state.
Now, there are a few stars which are much hotter than the Sun. The constellation of Orion has quite a few stars so hot that they emit mostly high-energy photons in the ultraviolet and blue.

Image of Orion courtesy of
Matthew Spinelli and Astronomy Picture of the Day
And during the day, the ordinary scattered sunlight that makes the sky blue is so bright that it overwhelms any aurorae that might be attempting to shine.
So, we need some form of energy that can reach the Earth's atmosphere AT NIGHT. Hmmm.
Q: Can you think of some sort of energetic particles
that might strike atoms in the Earth's atmosphere
at night?
How about -- the solar wind!
The Sun's gravity holds material in its atmosphere, preventing it from flying off into space -- under ordinary conditions. However, the magnetic field of the Sun can create storms in its upper atmosphere, heating the gas to temperatures of millions of degrees and causing it to flow out into space.

Animation of solar wind courtesy of
NASA GSFC/CIL/Krystofer Kim
Although they carry a lot of energy, the energetic particles of the solar wind spread out as they fly off into space. Their density is so small by the time they reach the Earth that only a few particles would smash into any patch of the atmosphere at a time, leading to a very faint, diffuse glow that would be impossible to detect.
Fortunately, the Earth has a strong magnetic field which can funnel the solar wind down into a relatively small region.

Animation courtesy of
NASA's Goddard Space Flight Center
In general, if a planet has a strong magnetic field, it will likely be easier to see any aurorae on that planet.
Let's play a little game. I'll walk through the Solar System, taking some of the major bodies in order of their distance from the Sun. Before I reach each planet, you should guess whether each one does, or does not, exhibit auroral activity. Perhaps the list of ingredients we've discussed earlier will give you some clues.
If you were standing on the surface and looking up, you wouldn't see any pretty lights in the sky. So, to my mind, this is not an aurora.

Gray et al., JGR Space Physics, vol 130 (2025)
This glow appears after strong solar wind events, and appears to be created by energetic protons from the Sun smashing into atoms in the lower levels of the Venusian atmosphere.
On one hand, it's such a faint signal, and so diffuse, that even the technical paper describing its discovery doesn't show a single picture of the planet to illustrate it. This phenomenon would definitely win no awards ...
But since the scientists studying it call it an "aurora", it counts!
In March, 2024, the Perseverance rover's cameras detected a faint, diffuse glow from oxygen atoms which had been excited by solar particles. In the pictures below, the left-hand panel (during an aurora) shows the tell-tale greenish glow of oxygen, which is missing from the ordinary Martian night sky shown in the right panel.

Image courtesy of NASA/JPL-Caltech/ASU/MSSS/SSI
This display looks a lot nicer in an artist's rendition ...

Image courtesy of Alex McDougal-Page/University of Strathclyde
The image below, based on images taken with the Hubble Space Telescope, shows the auroral ring around Jupiter's poles in ultraviolet light.

Image courtesy of NASA, ESA, and L. Frattare (STScI); Acknowledgment: NASA, ESA, J. Nichols (University of Leicester), and A. Simon (NASA/GSFC)
Because Jupiter's magnetic field is so strong, and because it interacts with the four large, close-by moons which surround it, the details of auroral emission from Jupiter can be very complex. If you'd like to dip your toes into those perilous waters, consider reading an article about the "footprint" of Callisto."
Saturn, like Jupiter, has both plenty of atmosphere and a strong magnetic field. It's no surprise that if one looks carefully at the polar regions of the planet, one sees -- an aurora!

Image courtesy of NASA, ESA, Hubble, OPAL Program, J. DePasquale (STScI), L. Lamy (Obs. Paris)
But not just the northern pole -- Saturn hosts an aurora at its southern pole as well!
Still, it does have a magnetic field, like the other giant planets, and a thick atmosphere, so one might expect to see some sort of aurora after a strong solar storm. And, indeed, we do, as these images from the Hubble Space Telescope reveal. But note that the glowing regions are much less well defined and symmetric than those on Jupiter or Saturn; perhaps that is a result of the unconventional spin and magnetic axis of this planet.

Image courtesy of ESA/Hubble & NASA, L. Lamy / Observatoire de Paris
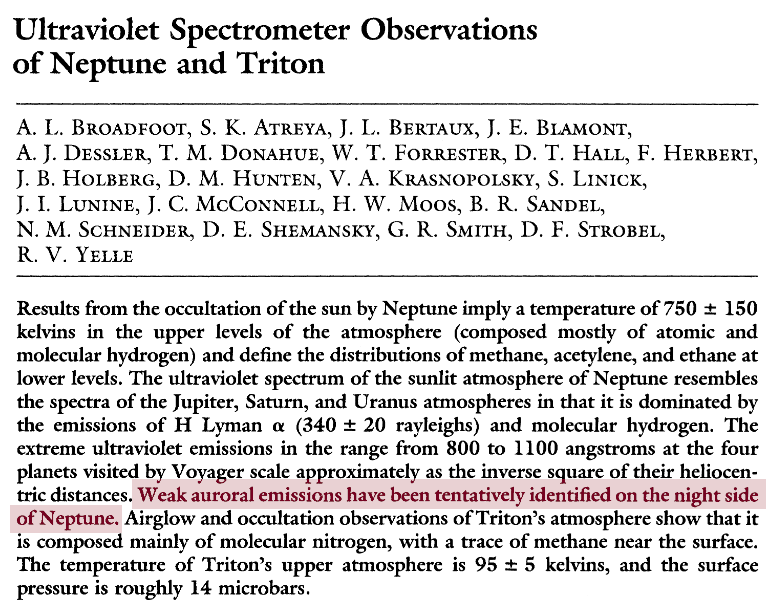
From the abstract of
Broadfoot, A. L., et al., Science, 246 (1989)
Over thirty years late, the James Webb Space Telescope finally was able to capture a picture of this auroral emission. Neptune, like Uranus, does not show the neat, circular patterns we see on Jupiter and Saturn; instead, its magnetic field brings energetic particles down into the atmosphere over almost an entire hemisphere! The auroral emissions are marked as greenish color in the right-hand panel below.
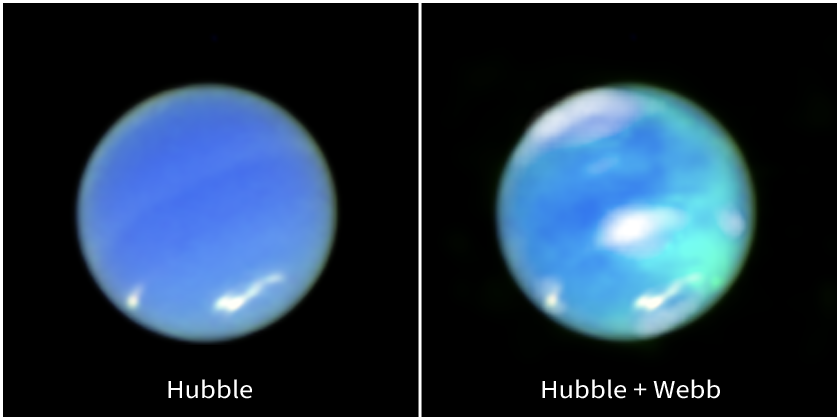
Image courtesy of NASA, ESA, CSA, STScI, Heidi Hammel (AURA), Henrik Melin (Northumbria University), Leigh Fletcher (University of Leicester), Stefanie Milam (NASA-GSFC)
Unlike the strong blue and ultraviolet emission we see in most planets' aurorae, this light actually appears in the infrared portion of the spectrum. It is produced by the very peculiar molecule H3+, composed of THREE hydrogen atoms joined together.
I haven't described all the methods that astronomers use to find planets around other stars. For a discussion of all those methods, in much greater detail, see the first of the references below.
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.