
Enhanced image highlighting water-rich surface regions Thanks to NASA/JHUAPL/SwRI. Click to enlarge.
Contents
On October 8, 2015, astronomers at the Southwest Research Institute announced that they had found evidence for water ice in large portions of the surface of Pluto.

Enhanced image highlighting water-rich surface regions
Thanks to NASA/JHUAPL/SwRI. Click to enlarge.
Wait a minute. Before we go any further, just how did they come to that conclusion. The New Horizons spacecraft flew past Pluto (and is still going, headed out of our solar system entirely). It didn't stop and land and grab samples of the surface. So, how can scientists tell that water is present?
A spectrograph takes light from a source and separates it by wavelength, so that the red light goes in one direction, the yellow light in another direction, the blue light in another direction, and so forth. There are two basic ways of dispersing light. You can pass it through a prism
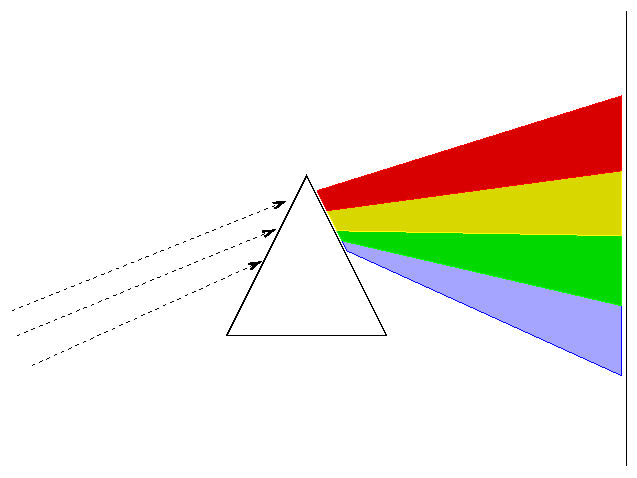
or you can bounce it off (or pass it through) a diffraction grating
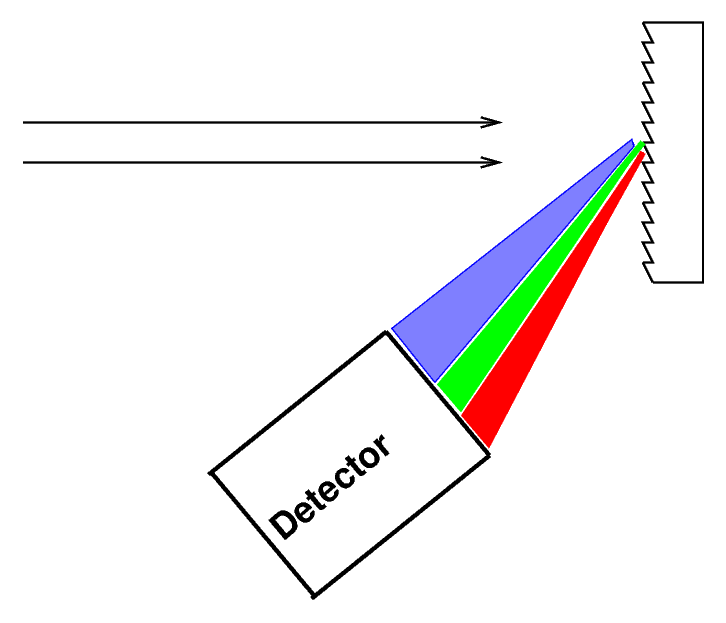
You can read about the physics of diffraction gratings if you want to understand exactly how and why they work.
Astronomers often place a slit over the focal plane of the telescope, centered on the object of interest.
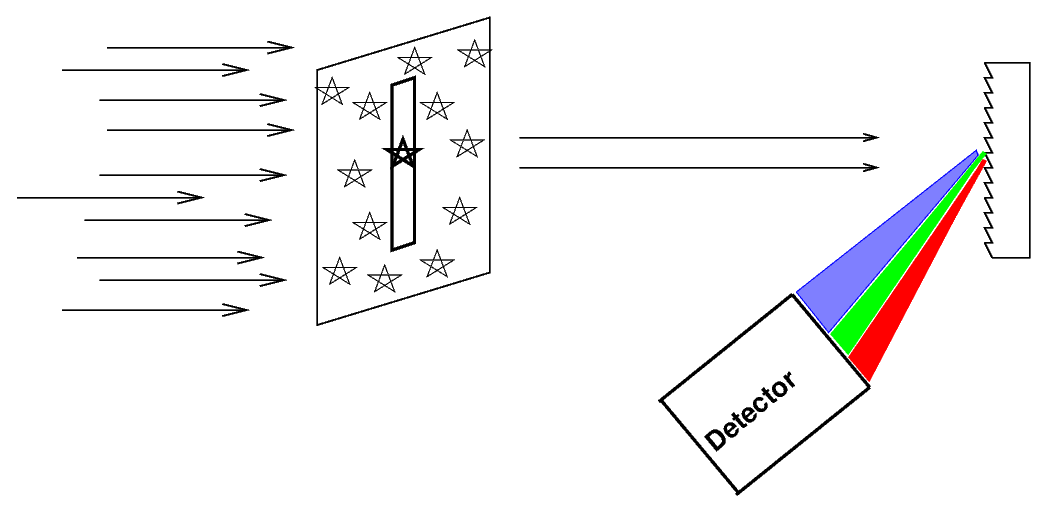
Only light which passes through this slit will strike the grating (or prism), giving the spectrum a characteristic shape: vertical lines on a long, horizontal canvas.
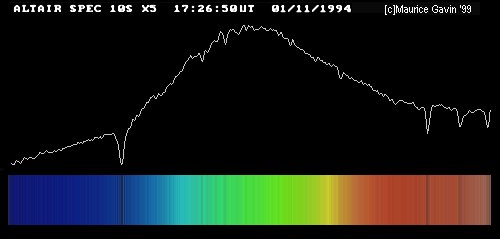
Image by Maurice Gavin, from the wpo-amateur spectroscopy web site.
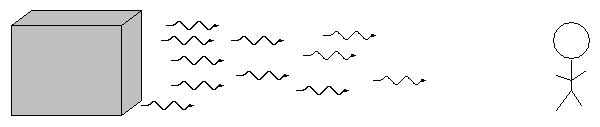
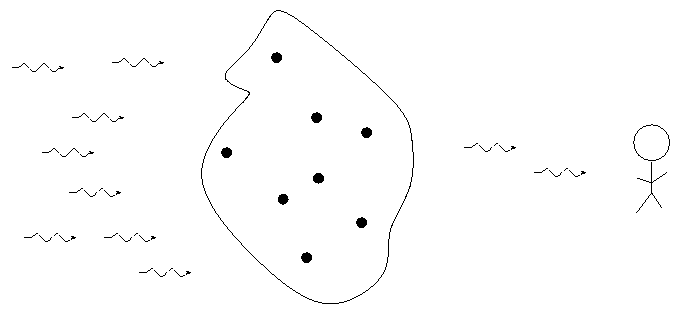
When we pass light from a source through a spectrgraph, we usually see one of three basic types of spectrum, depending on the nature of the source. German astronomer Gustav Kirchoff, working in the 1850s, figured out the reason for these different types of spectra. He explained the three basic types of spectra as coming from three different situations:
![]()
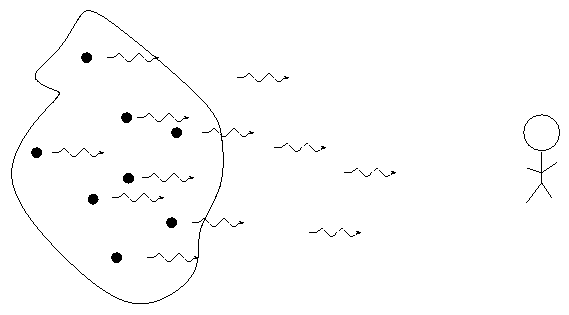
![]()
![]()
Each element generates its own unique set of wavelengths of emission or absorption.

We can use these patterns like fingerprints to identify the material which is emitting or absorbing light.
Okay, let's get back to Pluto. All our recent, detailed information comes from the New Horizons spacecraft. Launched in January, 2006, the relatively small probe took a slightly indirect route to the outer reaches of the solar system. As it zoomed past Jupiter in February, 2007, New Horizons "stole" some of the giant planet's orbital energy and angular momentum, shooting outward toward Pluto with an extra burst of speed.
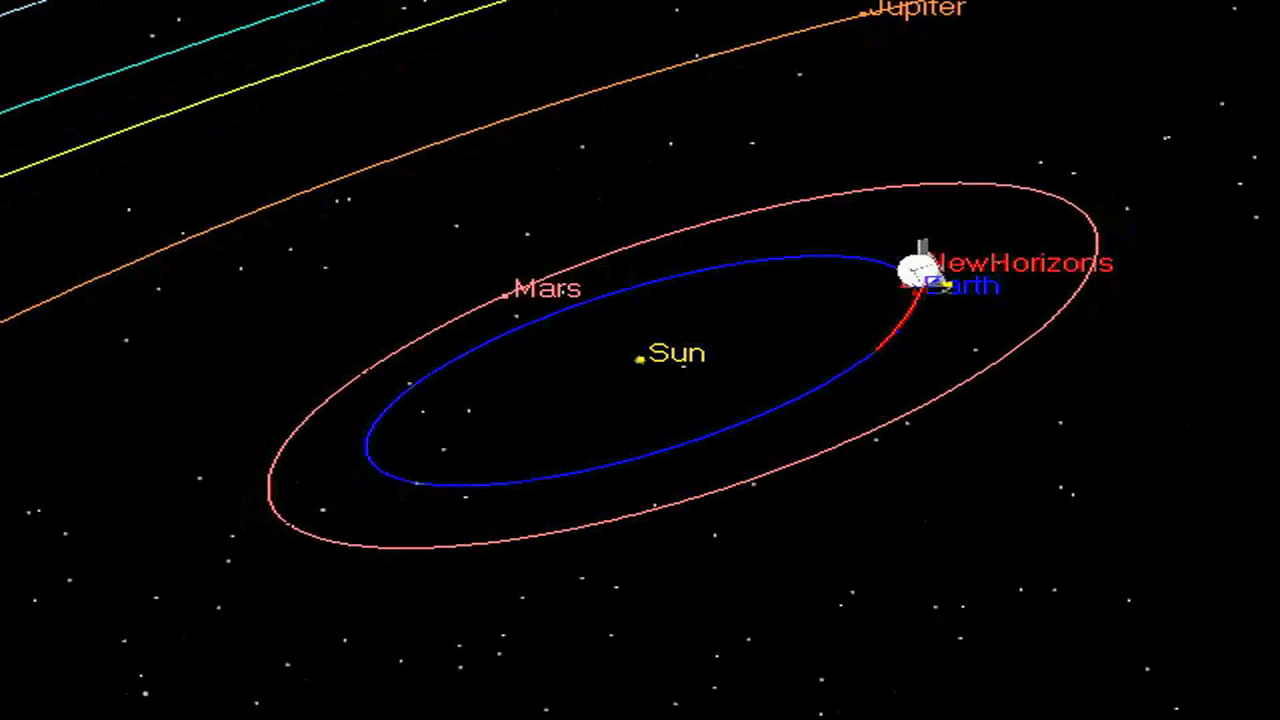
New Horizons Trajectory Animation
by Nader Mousa. Click on image to play movie.
As a result, New Horizons was able to reach the orbit of Pluto and its moon Charon in "just" nine years. Its speed was so great, however, that it wasn't able to slow down and enter an orbit around Pluto; instead, it had no choice but to fly past quickly and say good-bye after spending just a few hours making closeup measurements.
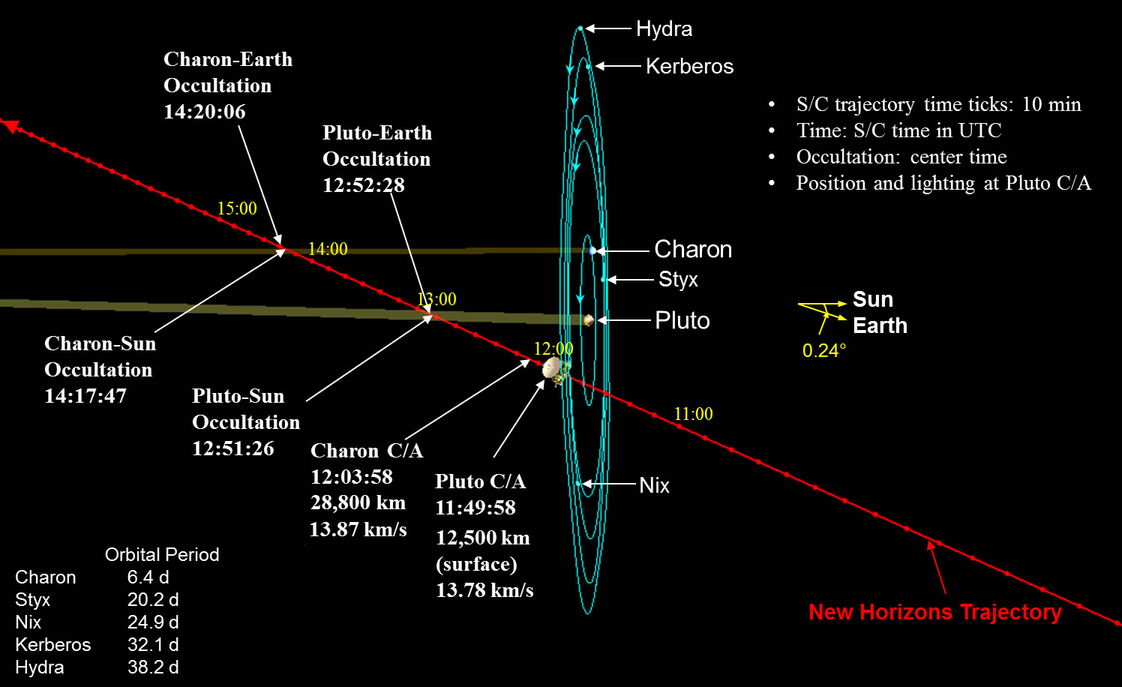
Image courtesy of
Matt Bergman
As a result, New Horizons had to record all the information gathered by its many instruments and save them in its memory. As time passes, the spacecraft is slo-o-o-owly transmitting its data back to Earth. In fact, right now (Oct 25), more than three months after the July 14 flyby, New Horizons is STILL sending some of that data. It won't finish for at least another few months!
Q: Why is it taking so long? Two main reasons --
can you guess them?
Put these two factors together, and the result is that New Horizons must send data slowly so that each bit can be picked out from the background noise correctly. The current datarate is roughly the same as, well, something you might remember from the past:

Can also go to
this URL on Youtube and FF to 00:06
In any case, astronomers working with New Horizons recently collected enough information to put together a color mosaic of the body: the picture below uses images taken through blue, red, and near-infrared filters to create a color composite. Note the areas which have an orangey-reddish color.

Image of Pluto released Sep 25, 2015 by NASA/JHUAPL/SwRI ,
composite of blue, red, near-infrared. Click to enlarge.
The Ralph spectrograph aboard New Horizons examined the surface at both optical and near-infrared wavelengths. In certain locations, it saw absorption bands in the near-infrared which are a signature of light reflecting off solid water ice.

Enhanced image highlighting water-rich surface regions
Thanks to NASA/JHUAPL/SwRI. Click to enlarge.
This was the first clear evidence for water ice on the surface of Pluto ... but not the first time water ice had been noticed in this neighborhood. Way back in 1994, astronomers using big telescopes on Earth were able to detect the same absorption bands in spectra of Pluto's moon, Charon.
Consider this abstract from a paper by Cruikshank et al. written in 1995:
Neptune's satellite Triton, and the planet-satellite binary Pluto and Charon, are the most distant planetary bodies on which ices have been directly detected. Triton and Pluto have very similar dimensions and mean densities, suggesting a similar or common origin. Through earth-based spectroscopic observations in the near-infrared, solid N2, CH4, and CO have been found on both bodies, with the additional molecule C02 on Triton. N2 dominates both surfaces, although the coverage is not spatially uniform. On Triton, the CH4 and CO are mostly or entirely frozen in the N2 matrix, while CO2 may be spatially segregated. On Pluto, some CH4 and the CO are frozen in the N2 matrix, but there is evidence for additional CH4 in a pure state, perhaps lying as a lag deposit on a subsurface layer of N2. Despite their compositional and dimensional similarities, Pluto and Triton are quite different from one another in detail. Additional hydrocarbons and other volatile ices have been sought spectroscopically but not yet have been detected. The only molecule identified on Pluto's satellite Charon is solid H2O, but the spectroscopic data are of low precision and admit the presence of other ices such as CH4.
Here are a few sample spectra acquired since that time, showing the strong water features in spectra of Charon; note particularly the dip at 1.65 micrometers.
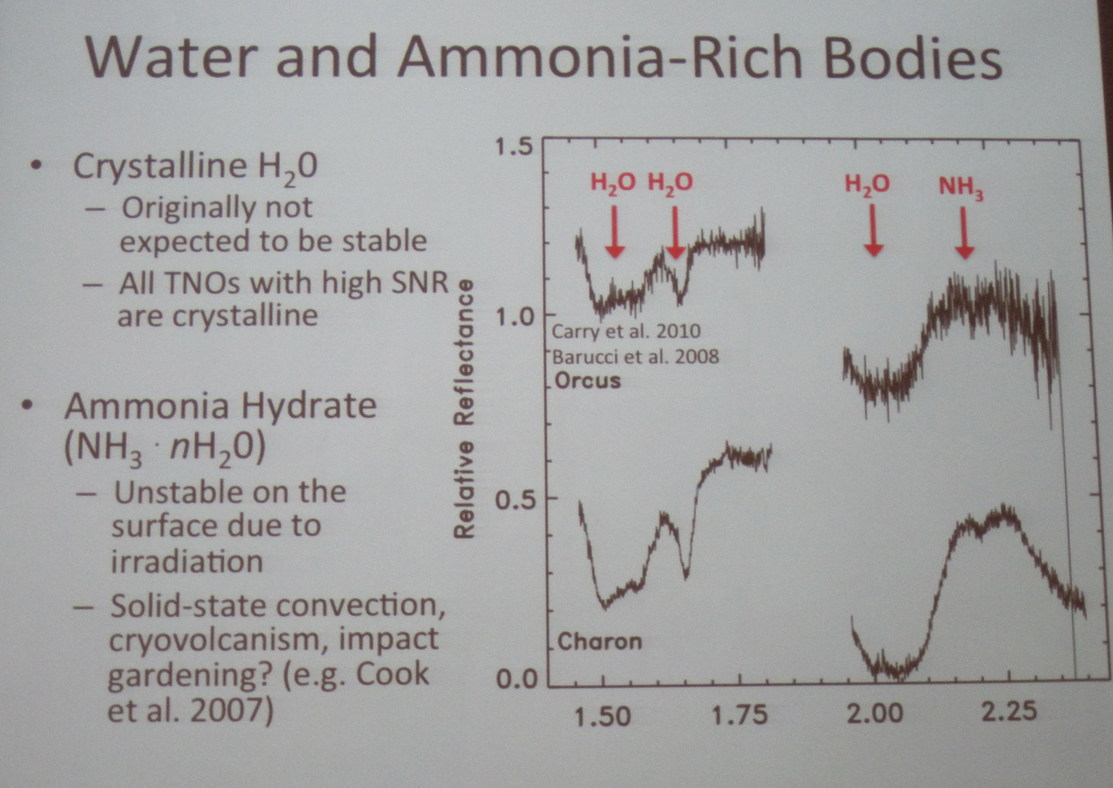
Figure from talk by Francesca DeMeo (MIT) titled
"Near-Infrared Spectroscopic Measurements of Charon with the VLT."
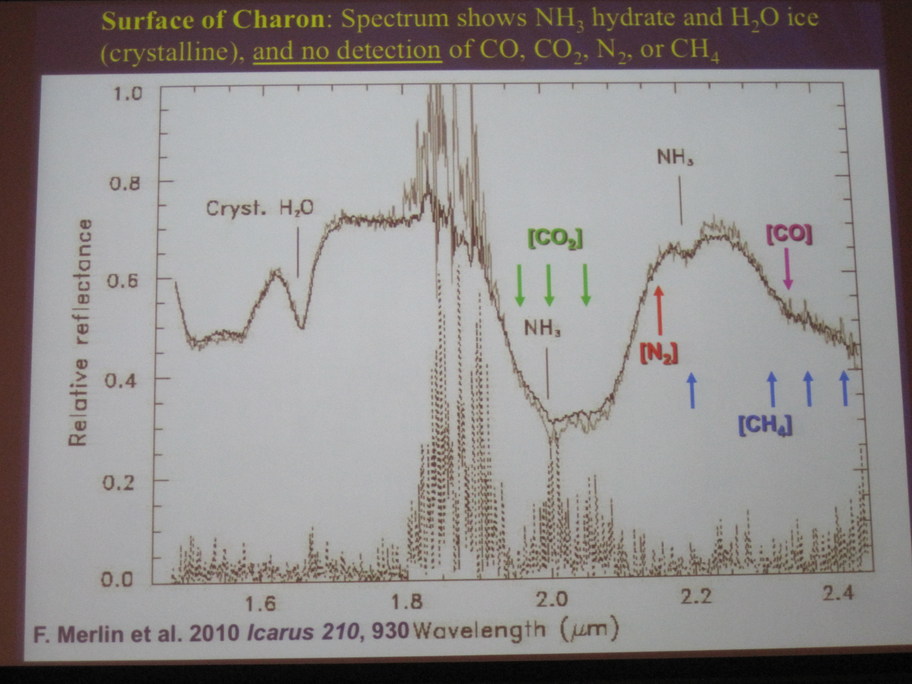
Figure from
Merlin et al., Icarus 210, 930 (2010)
presented in
a summary talk by Dale Cruikshank
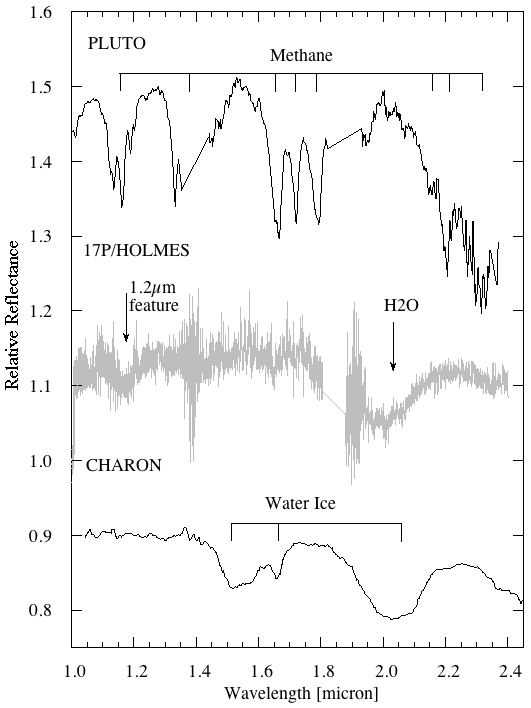
Spectra of Pluto (top) and Charon (bottom), originally published
in
Brown and Calvin, Science, 287, 107 (2000) ,
and reproduced to compare with Comet 17P/Holmes by
Yang, Jewitt, and Bus (2009).
Water ice may make up such a large fraction of the crust of Pluto that it may be responsible for the bulk of these mountains:
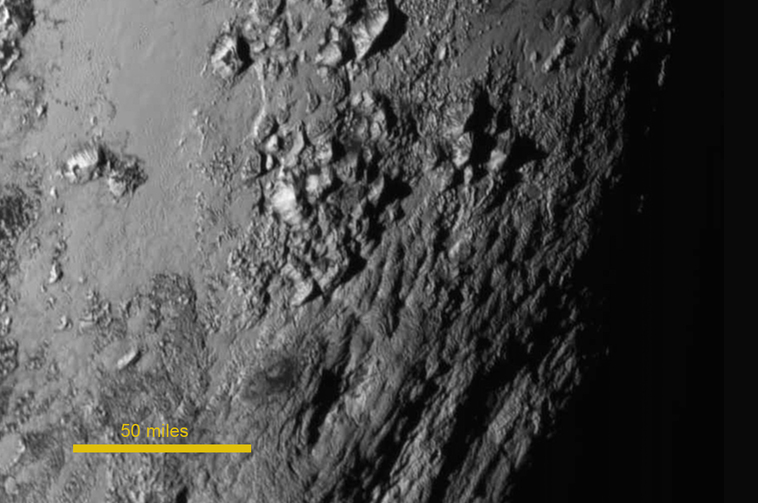
Mountains mostly like made of water ice, up to 3500 meters tall,
on the surface of Pluto.
Image courtesy of NASA-JHUAPL-SwRI
Click to enlarge.

Backlit view of the Norgay Mountains on Pluto
Image courtesy of NASA-JHUAPL-SwRI
Click to enlarge.
So we know that not only Charon, but also Pluto, has large amounts of water ice in its surface. It may sound surprising ... but actually, it makes perfect sense. We should EXPECT there to be quite a bit of water ice in the cold outer regions of our solar system.
Q: Why should we EXPECT water out there?
Well, if you go back, way back, to the infancy of the universe, just after the Big Bang, you find yourself in a very hot, very dense soup. Over a period of several minutes, nuclear reactions create a set of particles which will one day become the nuclei of chemical elements. Just two of these elements are by far the most common.
Fast forward 13 billion years, and the chemical makeup of material in our Milky Way Galaxy has changed just a bit. Thanks to fusion in the centers of stars, some heavier elements have been created, and then -- due to stellar winds and explosions -- spewed back out into space. If we take a census of the atoms in the Milky Way as a whole, we find something like this:
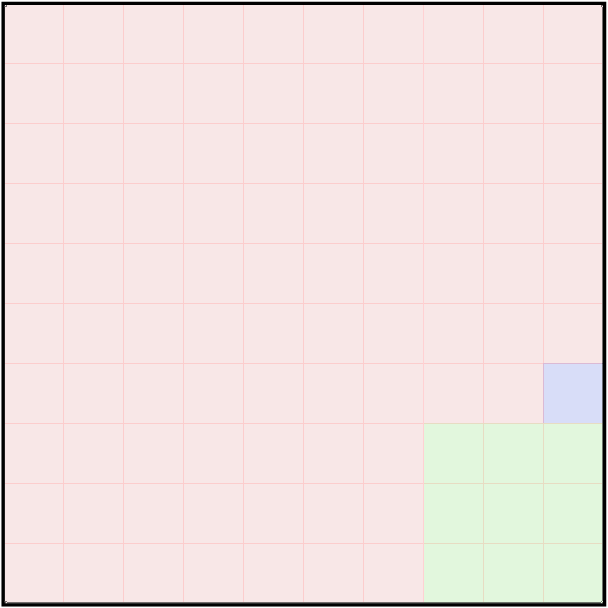
Q: What are those two most common elements?
Right. Hydrogen and helium. In our solar system, these two light elements account for over 99 percent of all the atoms.
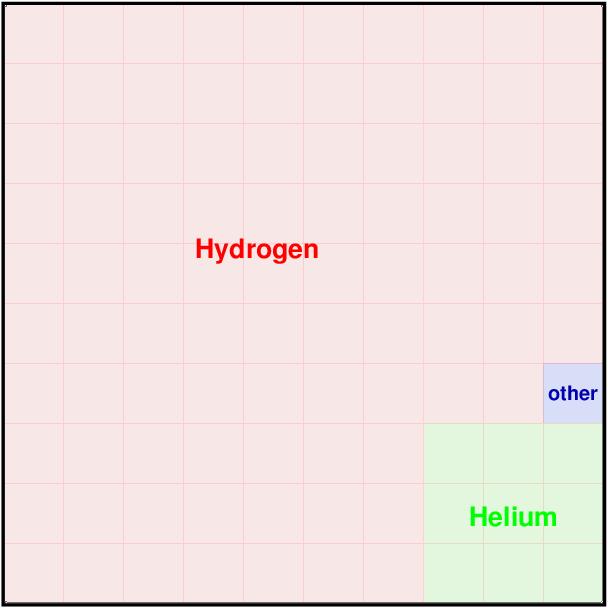
But what about the remaining chemical elements? Even if they make up less than 1 percent of the matter in our Galaxy, surely some are more common than others.
Q: What are these most common "heavy" elements?
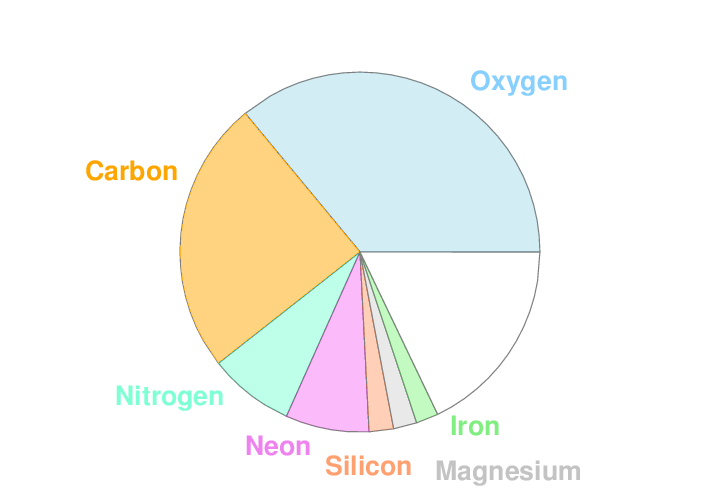
The three most common are oxygen, carbon, and nitrogen.
Now, remember that hydrogen and helium atoms will far outnumber these "metals" (yes, astronomers call oxygen and nitrogen "metals". We're a strange bunch). Helium is a noble gas, and doesn't combine with other elements, or even with itself. So, if any atoms are going to combine to form molecules, the most common are going to be:
hydrogen with hydrogen ---> H2
hydrogen with oxygen --->
hydrogen with carbon --->
hydrogen with nitrogen --->
Hydrogen molecules remain a gas, even at the ultra-cold temperatures near Pluto, roughly T = 40 Kelvin = -233 Celsius = -387 Fahrenheit; so, solid bodies like Pluto and Charon won't be made of hydrogen.
The most common substances which are solid in these frigid, dark areas are the molecules listed above.
And THAT is why we should not be surprised to find that Pluto has lots of water ice on its surface (and probably down below, too).
On September 28, 2015, NASA announced that it had strong evidence that liquid water flows on today's Mars. Hmmm. Haven't we heard something like that before?
Well, the key words in that announcement are liquid ... flows and today's . Let's investigate this claim, shall we?
Sherman, set the Wayback Machine for 1963. In that year, astronomers on Earth, using large ground-based telescopes and spectrographs, detected absorption lines due to water vapor in the atmosphere of Mars; see the Detection of Water Vapor on Mars by Spinrad, Munch and Kaplan (ApJ 137, 1319, 1963),
In 1976, the US sent two Viking spacecraft to Mars. Each Viking split into two units when it reached the planet, leaving one section in orbit while the other landed on the surface. Pictures sent back from the surface showed clear evidence for misty water vapor on occasional warm days

Image of frost on the ground
from the Viking 2 landing site,
from
The Martian Landscape (NASA SP-425)
as did photographs taken from the orbiters:
Early Morning Clouds in Noctis Labyrinthus
from
Viking Orbiter Views of Mars (NASA SP-441)
and
APOD 2001 Apr 17
So, we have had clear evidence for decades that there is water vapor near the Martian surface.
In addition, photographs of the polar ice caps of Mars, taken from Earth

Image courtesy of
Don Parker
and from Mars orbit
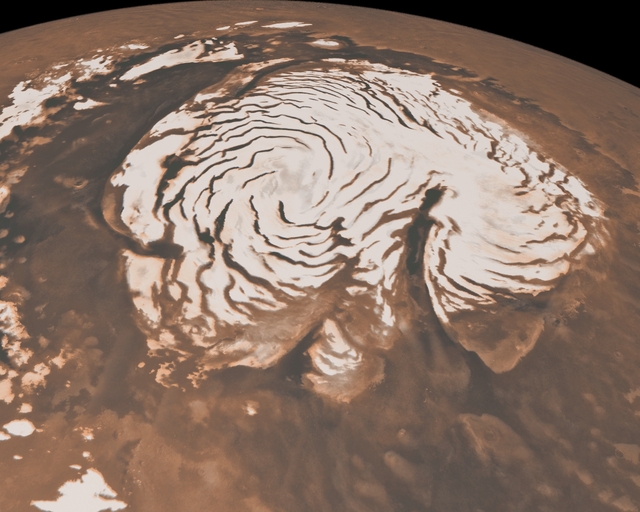
Image courtesy of
NASA/JPL-Caltech/MSSS.
Click to enlarge.
and from the surface, thanks to the Mars Polar Lander

Image courtesy of
Marco Di Lorenzo, Kenneth Kremer,
Phoenix Mission, NASA, JPL, UA, Max Planck Inst., Spaceflight
and
APOD for 2008 Nov 12
make plain the presence of frozen water ice (yes, mixed together with frozen carbon dioxide, it's true) on Mars.
What about liquid water on Mars? Images taken from orbiting spacecraft have shown over and over again surface features which were undoubtedly created by flowing liquid water ... but long, long ago.
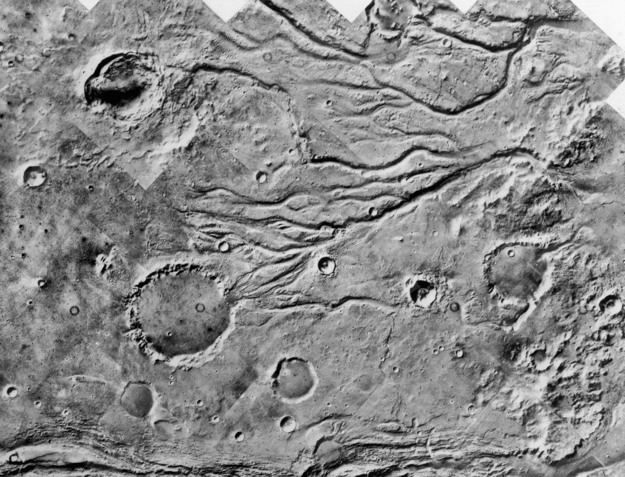
Viking Orbiter mosaic
courtesy of
NASA and Goddard Earth Sciences Data and Information Services Center
Click to enlarge.
But -- although there were tantalizing hints of LIQUID water on Mars at the CURRENT TIME, the evidence was never very strong ... until now. Scientists had for several years been studying dark streaks which appear near steep cliffs, called "recurring slope lineae", or RSL for short. These streaks turned dark and light, dark and light, in tune with the changing seasons.

Warm Season Flows on Slope in Horowitz Crater.
Image courtesy of
Wikipedia and
NASA/JPL-Caltech/Univ. of Arizona
The key new piece of information was provided by the CRISM spectrograph on board the Mars Reconnaissance Orbiter. CRISM repeatedly measured the spectrum of the surface near these dark streaks, and found that features of hydrated salts appeared when the streaks were at their darkest and widest. Conclusion: the dark streaks are caused by salty brine
In other words, (very salty) liquid water on Mars now!