 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
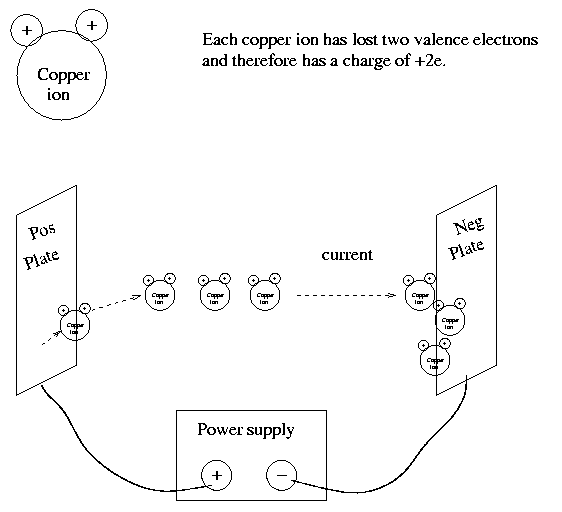
In this experiment, a current flows through a solution of copper sulfate. Copper ions pop off the surface of the anode (positively charged plate), flow through through the liquid, and stick onto the surface of the cathode (negatively charged plate). Each copper ion has lost two valence electrons, and so has a charge +2e (where e is the size of the charge on one electron).

We describe the current using amps. One amp is equal to one Coulomb of charge passing through a given point each second. So, if one Coulomb of charge sticks to the cathode each second, the current must be one amp.
Your job is to figure out how many Coulombs of charge are carried by each electron; or, in this particular experiment, by a positive charge equal in size to an electron's negative charge. The way to do it is to figure out two different things:
Once you know how many Coulombs have moved, and how many electron-sized charges have moved, you can calculate how many Coulombs per electron-sized charge.
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.