Remember that 10 Angstroms = 1 nm, so 4000 Angstroms = 400 nm = blue light ...

Remember that 10 Angstroms = 1 nm, so 4000 Angstroms = 400 nm = blue light ...
n energy (eV)
-------------------------
1 -5.14
2 -3.04
3 -1.96
4 -1.52
-------------------------
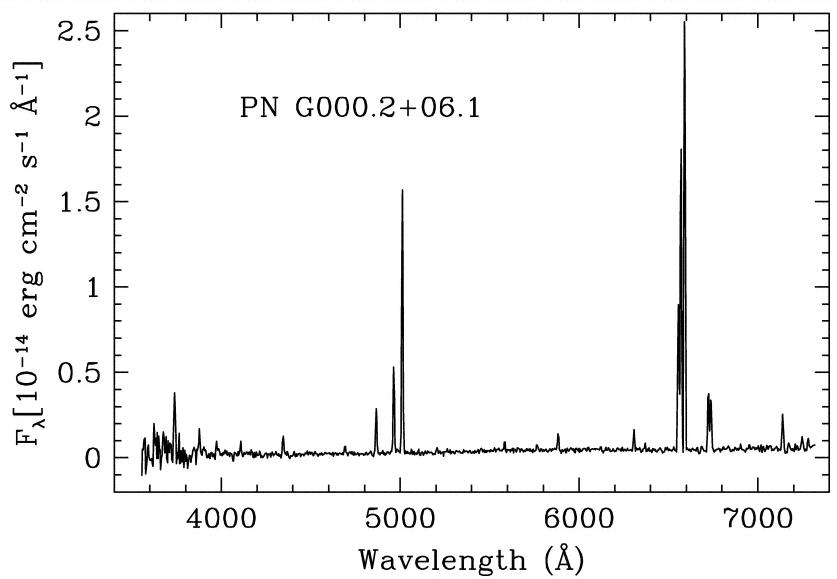
One of the strongest lines in the spectrum above is due to a transition in sodium atoms. Which one is it? What is the wavelength, and what are the levels involved?