 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
This project must be done by individuals. It must be submitted via myCourses by 5 PM on Dec 1, 2020.
The STATSTAR program creates an output file which lists the properties of a large number of thin shells of material inside a star. Let's use some of those tabulated values to compute the total "thermal energy" of the gas inside a Sun-like star.
By "thermal energy", I mean "the kinetic energy of all the particles in the gas." As you may recall, the thermal energy of particles in a gas depends solely on the temperature of the gas:
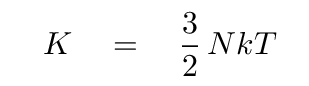
So, if you can figure out the number of particles N in a shell of the model, and the temperature of the gas in that shell, then you can compute the thermal energy of that shell. Add up the thermal energy of all the shells in the star, and voila! you have the total thermal energy.
There are two tricky bits to this project.
But in order to figure out the number of particles, you need to do some extra work. We will make the following assumptions:
As a warmup, please work out the following, step by step, and include it with your project. Consider 1 kg of gas with the above composition.
Good. You have just calculated the number of particles inside every kg of ionized gas in the star. That means that you can convert the MASS of each shell into the total number of particles N of each shell.
Here's the datafile you should use. It's the result of running STATSTAR on a star of 1 solar mass, with the chemical composition given above, and an effective temperature of 5500.2 Kelvin. There are two versions with the same numbers -- use whichever one is easier for you to read into a program.
Your task is to compute the following quantities:
Write down this total mass.
L = 0.86071 Lsolar = 3.27 x 1026 J/s
If an evil wizard waved his staff and caused all fusion
in the star's core to stop,
how long could the star continue to radiate the
same luminosity, if it used up all the thermal energy
stored in the gas?
Could this stored thermal energy power the star for
the actual age of the solar system?
Scan/photograph all your work, which must include the "warmup" exercise, as well as the answers to items A, B, C. Create a single PDF file with all the material, and submit it via myCourses.
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.