 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.




Q: What is the first derivative of psi? Q: What is the second derivative of psi? Q: Can you write the Schroedinger equation again, substituting in the values of the derivatives?


Q: Using the first equation, find the value of "a".
Evaluate it and find its value in meters.

However, note that this does NOT mean that the electron is always exactly the Bohr radius, as it would be in the Bohr model. Schroedinger's model of the atom provides only relative probabilities for the location of the electron. More on this tomorrow ....
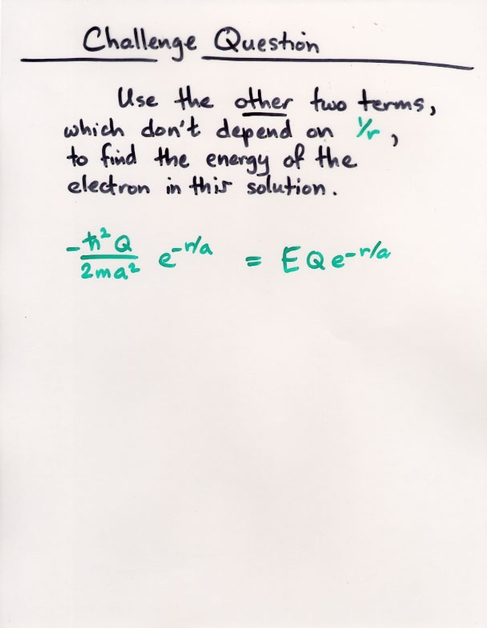
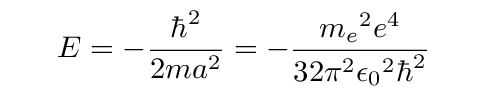
That should look familiar ...

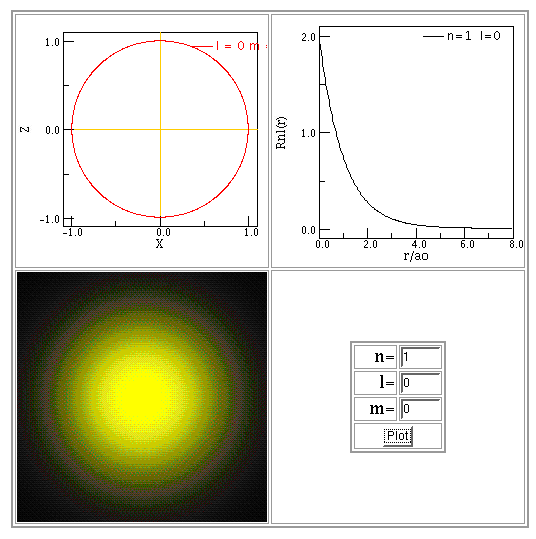
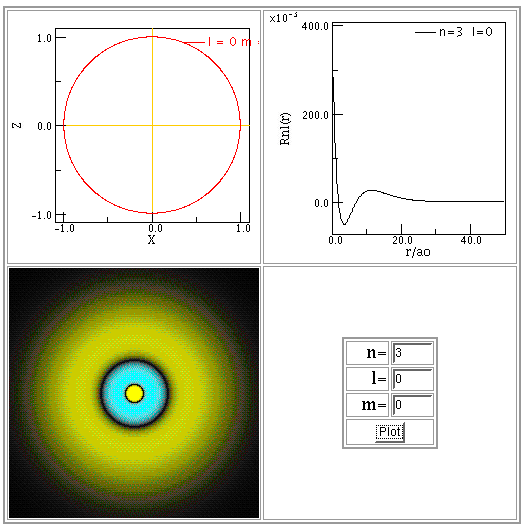
For example, here are illustrations of the solutions in the ground state, n = 1, first excited state, n = 2, and the second excited state, n = 3. Note that the size of the graphs changes!

 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.