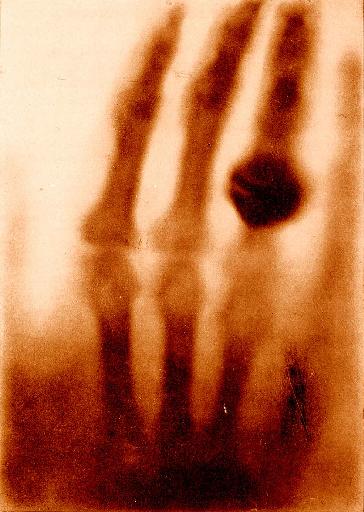
Thanks to Dream Anatomy for a copy of Roentgen's X-ray of his wife's hand.
How are new elements discovered? These days, it is through deliberate, concerted effort in a known direction: the only empty spots in the periodic table are all at the high end, at atomic numbers above 105 (or 108, or whatever the current value is). Physicists know that if they bombard heavy elements with energetic nuclei, they may very occasionally create a very heavy nucleus; and, if such nuclei should last long enough to build up a macroscopic sample of material, they may measure the properties of a new element. It is hard work, but follows a clear path with a well defined goal.
Back in the old days, however, life was not so simple. The periodic table was still a work in progress: there were plenty of gaps and holes, as well as puzzling inconsistencies. More important, there was no theory to guide scientists in their work: the nature of atoms and nuclei was not yet understood at the turn of the twentieth century. Chemists and physicists did not know that there were exactly 30 distinct elements between hydrogen and zinc, or that helium would have exactly five siblings among the (stable) noble gases.
Let us look at the discovery of the elements polonium and radium: they were tentatively announced by the team of Pierre Curie, Marie Curie, and Gustave Bemont in 1898. It was four more years, however, until Marie Curie was able to isolate a pure enough sample of radium to measure some of its properties.
In 1895, Wilhem Conrad Roentgen, a physicist working at the Unversity of Wurzburg in Germany, was investigating the properties of a "Crookes tube": an evacuated glass tube through which a strong electric current is passed at high voltage. He noticed some curious phenomena which were somehow connected to the operation of the Crookes tube, but took place far from it.

Thanks to Dream Anatomy for a copy of
Roentgen's X-ray of his wife's hand.
Roentgen realized that invisible rays were coming from the Crookes tube, flying through the air, and interacting in novel ways with a wide variety of materials. He named the rays X-rays.
People around the world were excited by the discovery of these rays, and the fascinating properties they had. Was it possible that there could also be other "rays" in the world?
Just a year later, French physicist Henri Bequerel noticed a peculiar little event: he left a small piece of uranium (wrapped up inside a piece of paper) sitting on a photographic plate in a drawer for several weeks. When, on a lark, he developed the plate, he found a dark spot at the location which had been directly under the uranium. Somehow, the uranium had exposed the photographic plate, even though it had been covered in opaque material. Could this be another sort of invisible ray?
In 1897, a graduate student at the University of Paris named Marie Curie decided to investigate one aspect of this new phenomenon for her doctoral dissertation. She was twenty-nine years old and had just given birth to her first child, Irene, during September of that year. "What," she asked, "was the nature and origin of this radiation?" She would continue this investigation for the rest of her life. Her husband, Pierre Curie, a physicist at the University, was her partner in the work until his death in 1906.
The first step was to look at the source of the radiation. It was known that materials containing compounds of uranium and thorium were radioactive (a term coined by Marie Curie). The Curies decided to look harder ...
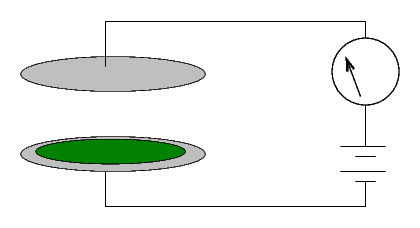
How can one measure radioactivity in a quantitative manner? One instrument used by the Curies was a simple device:
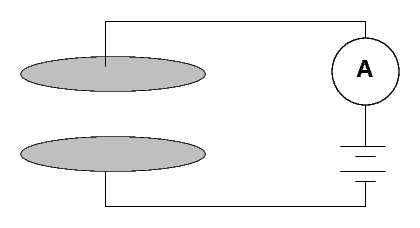
Two metal plates are separated by a gap of a few centimeters of air. Marie Curie described hers as follows:
(Diameter of the plates 8 cm; distance 3 cm.) A difference of potential of 100 volts was established between the plates. The current which passed through the condenser was measured in absolute value by means of an electrometer and a piezo-electric quartz.When a voltage is applied across the plates, no current runs. Why not?From "Rayons Emis par les Composes de L'Uranium et du Thorium," Comptes rendus, Vol 126, April 1898
Because air isn't a conductor, of course.
However, if one can somehow change the air so that it WILL conduct electricity, then the size of the current will indicate the conductivity (or resistivity) of the air. Bombarding the air with energetic particles can ionize some of the molecules, turning them into charged entities
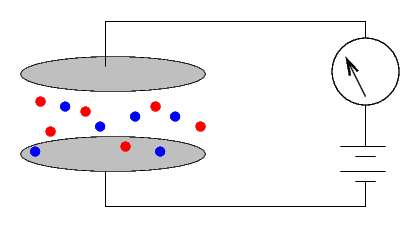
which will carry a current.
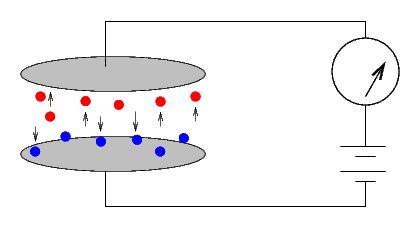
The Curies would simply cover one of the two plates with some material. If no current ran through the system, it was inert ...
... but if a current did run, then the material was radioactive. The size of the current provided a quantitative measure of the radio-activity of the substance.
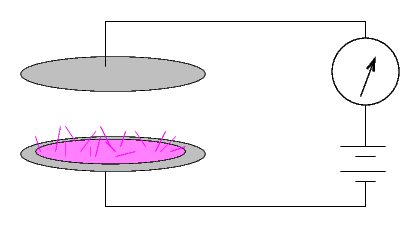
This setup produced currents which were very small: a sample of pure uranium gave a current of 24 picoamps (that's 24 x 10-12 amperes), for example.
Marie Curie reported a very puzzling result: pure uranium and thorium did not produce as much current as certain ores (pitchblende and chacolite) from which they were refined.
| Material | picoamps |
| Uranium | 24 |
| Black oxide of uranium | 27 |
| Hydrated uranic acid | 6 |
| Pitchblende from Johanngeorgenstadt | 83 |
| Pitchblende from Joachimstahl and from Pzibran | 67 |
| Natural chacolite | 52 |
| Artificial chacolite | (see below) |
Chacolite is a mixture of copper and uranium phosphates. When Marie Curie made her own "artificial chacolite" by combining pure samples of copper and uranium with other materials, she found the resulting compound to have no more radioactivity than other uranium compounds.
So, why were some of the raw materials stronger sources of radiation than pure uranium and thorium? The Curies speculated that there might be an extra, very strongly radioactive component in the raw mixtures which was eliminated by chemical processing.
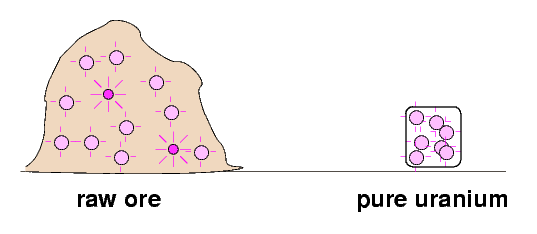
They set out to find this postulated, very radioactive substance which was hiding in the raw ores.
During 1898, the Curies continued their chemical experiments, trying always to identify the portion of the uranium ore which was most strongly radioactive. In December, 1898, they announced that there were apparently two highly radioactive substances hidden in the ore. They explain:
Two of us have shown that by purely chemical processes one can extract from pitchblende a strongly radioactive substance. This substance is closely related to bismuth in its analytical properties. We have stated the opinion that pitchblende may possibly contain a new element for which we have proposed the name polonium.The investigations which we are now following are in accord with the first results obtained, but in the course of these researches we have found a second substance strongly radioactive and entirely different in its chemical properties from the first. In fact, polonium is precipitated out of acid solutions by hydrogen sulphide, its salts are soluble in acids, and water precipitates them from these solutions; polonium is completely precipitated by ammonia.
The new radioactive substance that we have just found has all the chemical aspects of nearly pure barium: It is precipitated neither by hydrogen sulphide, nor ammonium sulphide, nor by ammonia; the sulphate is insoluble in acids and water; the carbonate is insoluble in water; the chloride, very soluble in water, is insoluble in concentrated hydrochloric acid and in alcohol. Finally this substance shows the easily recognized spectrum of barium
We believe nevertheless that this substance, although constituted for the greater part by barium, contains in addition a new element which gives it its radioactivity and which moreover is very close to barium in its chemical properties ...
From Sur Une Nouvelle Substance Fortement Radio-Active, Contenue Dans La Pitchblende by M. P. Curie, Mme. P. Curie and M. G. Bemont, Comptes rendus, Vol 127, Dec 1898.
The barium-like substance was by far the strongest source of radioactivity in the ores: they measured an activity level nine hundred times higher than that of uranium!
Let's stop for a moment to look at the actual levels of radio-activity for samples of uranium and radium. Over periods short compared to the half-life of two substances, the relative number of decays is equal to the inverse ratio of their half-lives. Suppose we have two samples of material, uranium and radium, with an equal number of atoms in each.
Q: What is the half-life of the most common
isotope of uranium, U-238?
Q: What is the half-life of radium-226?
(This is produced in the sequence of decays
starting from U-238)
Q: What is the ratio of (number of decays from
the sample of radium during a day) to
(number of decays from the sample of uranium
during a day)?
Q: Was the Curie's sample of radium pure
at this point in 1898?
A spectrum of this very active substance, taken by Eugene Demarcay, showed a number of strong lines due to barium and lead; but there was also a strong line at a wavelength of 3814.8 Angstroms. Demarcay wrote:
It does not seem possible to me that this line can be attributed to any known element ... Neither barium nor lead from elsewhere [i.e. from sources other than the Curies' material], as I have assured myself, give any line which coincides with it.From Sur le Spectre d'une Substance Radio-Active by M. Eug. Demarcay, Comptes rendus, Dec 26, 1898.
The NIST Atomic Spectra Database shows a line of Ra II (singly-ionized radium) at 3814.42 Angstroms.
The Curies suggested that this very radioactive substance might join polonium as a new element:
The various reasons which we have just enumerated lead us to believe that the new radioactive substance contains a new element to which we propose to give the name radium.From Sur Une Nouvelle Substance Fortement Radio-Active, Contenue Dans La Pitchblende by M. P. Curie, Mme. P. Curie and M. G. Bemont, Comptes rendus, Vol 127, Dec 1898.
The Curies paid out of their own pockets for bags of discarded slag to be shipped to them in Paris from the mine at St. Joachimsthal (in Bohemia, northeast of Berlin).
Where could the Curies perform their chemical extractions? The University of Paris offered them an abandoned shed in one of the courtyards; the School of Medicine had used it as a dissecting room in the past, but it was considered unfit for cadavers in 1898. A visitor to this shed described it as follows:
I knocked at a door chosen at random and enetered a laboratory of astonishing simplicity: the floor was of rugged beaten earth, the walls of ruined plaster, the ceiling of rather shaky laths, and the light came in weakly through dusty windows. .... It was cold. Drops of water were falling from a tap. Two or three gas burners were alight."Echo de Paris", by Paul Acker, quoted in Madame Curie by Eve Curie.
This image from the Fall 2002 AIP Newsletter shows a portion of the interior, with large carboys holding liquids used in the fractionation process.

The shed had no chimneys to carry off noxious fumes; the Curies could only open the door to increase the breeze through the windows. It was hot in the summer and frigid in the winter; one of Marie Curie's notebook page for February 6, 1898 includes the words "Temperature here 6.25 !!!!!!!!!!" (that's 6.25 degrees Celsius, about 44 Fahrenheit).
German chemist Wilhelm Ostwald visited the shed to see how the radioactive elements were being isolated. He wrote:
At my earnest request, I was shown the laboratory where radium had been discovered shortly before.... It was a cross between a stable and a potato shed, and if I had not seen the worktable and items of chemical apparatus, I would have thought that I was been played a practical joke.This anecdote taken from an article about Marie Curie's Nobel Prize
Marie Curie later wrote of her struggle to continue their investigations under the harsh conditions.
We had no money, no laboratory and no help in the conduct of this important and difficult task. It was like creating something out of nothing ... And yet it was in this miserable old shed that the best and happiest years of our life were spent, entirely consecrated to work. I sometimes passed the whole day stirring a mass in ebullition, with an iron rod nearly as big as myself. In the evening I was broken with fatigue.

From
The Discovery of Radium page at
Lateral Science.
For month after month, the Curies persisted in their quest to isolate the new elements. They were joined by Andre Debierne, who concentrated on the portions of material which were precipitated out of acid solutions by ammonia compounds. In 1899, he announced the isolation of a third radioactive element, similar in its properties to titanium. He named this material actinium.
But radium proved more difficult to isolate. For four years, Marie Curie continued to stir, sift, heat, cool, dissolve, precipitate and treat the radium-bearing portions of the discarded ore. Over this time, she processed about seven tons of slag from the mines in Joachimsthal, largely by her own hands. In August, 1902, she finally managed to create a relatively pure sample of radium chloride, one decigram by mass.
radium chloride 0.1 g --------------- = ------------ = 1 part in 80 million original mass 8,000,000 g
With this sample, she was able to determine the atomic weight of radium to be 225, showing clearly that it must be a new element.
One year later, on June 25, 1903, the University of Paris approved Marie Curie's thesis research, conferring on her the title of Doctor of Physical Science, with high honors. Two of her three examiners would go on to win Nobel Prizes over the next five years ... but Marie beat them to it.
In December, 1903, Marie and Pierre Curie were awarded a portion of the Nobel Prize in Physics, together with Antoine Henri Becquerel. Neither was able to travel to Sweden for the ceremonies, as they were both in poor health (Marie had miscarried a child in August). They did make it to Stockholm eighteen months later, where Pierre Curie gave a Nobel lecture.
Less than a year after this triumph, he was killed in a traffic accident, leaving behind Marie and her two young daughters. Marie would go on to win a second Nobel Prize in Chemistry in 1911. Older daughter Irene continued her family's research on radioactive substances, winning the 1935 Nobel Prize in Chemistry with her husband, Frederic Joliet, for work on the transmutation of elements.