 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Back in the 1890's, Dutch physicist Pieter Zeeman
had an idea: was it possible that magnetic fields could affect light?
In consequence of my measurements of Kerr's magneto-optical phenomena, the thought occured to me whether the period of the light emitted by a flame might be altered when the flame was acted upon by magnetic force. It has turned out that such an action really occurs. I introduced into an oxyhydrogen flame placed between the poles of a Ruhmkorff's electromagnet, a filament of asbestos soaked in common salt. The light of the flame was examined with a Rowland's grating. Whenever the circuit was closed both D lines were seen to widen.
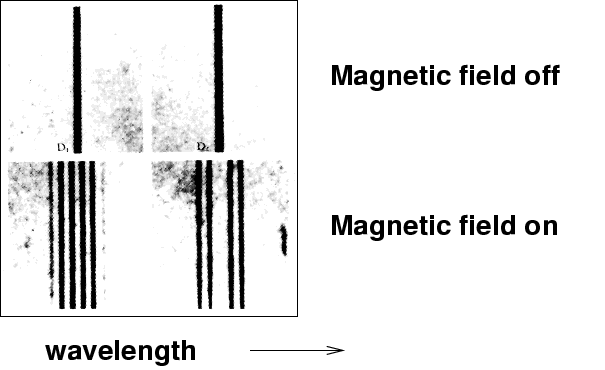
The photo above shows spectra of the sodium flame: wavelength runs along the X-axis.
What's going on?

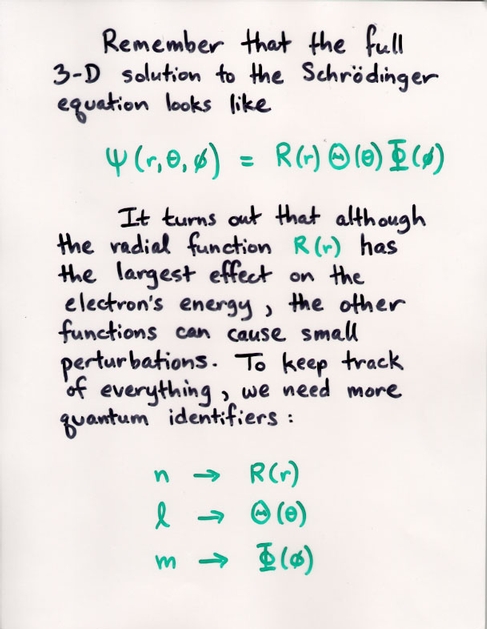
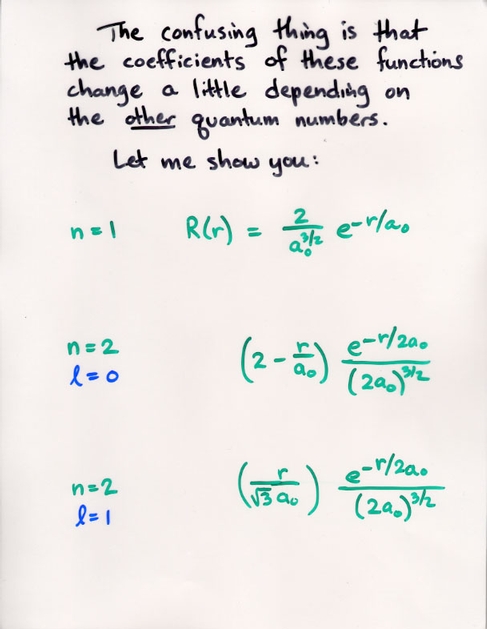
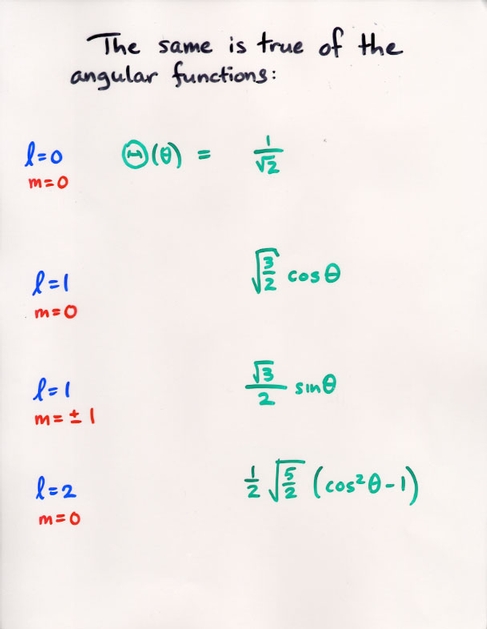



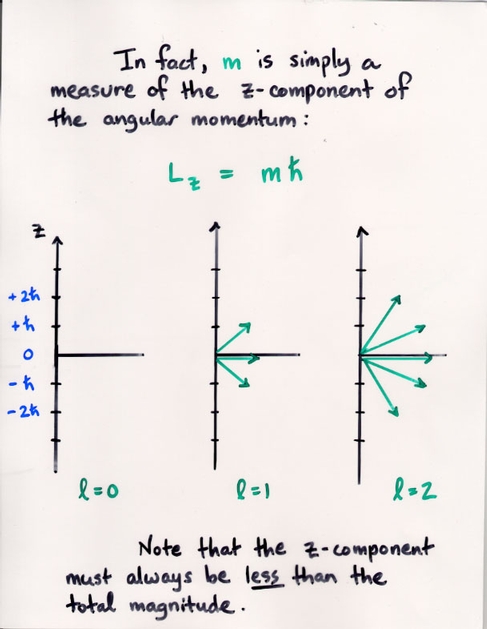
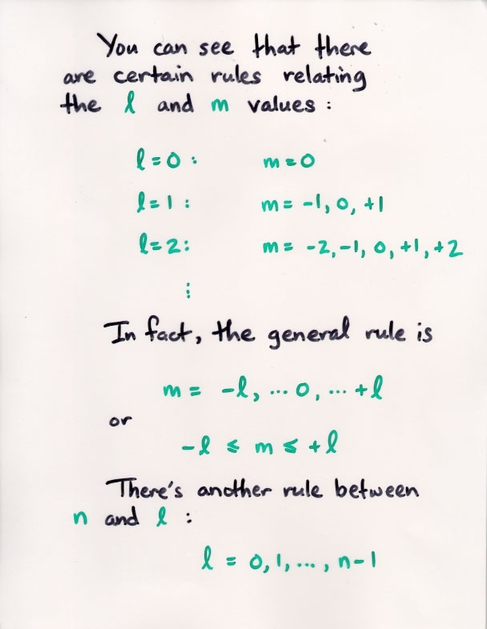
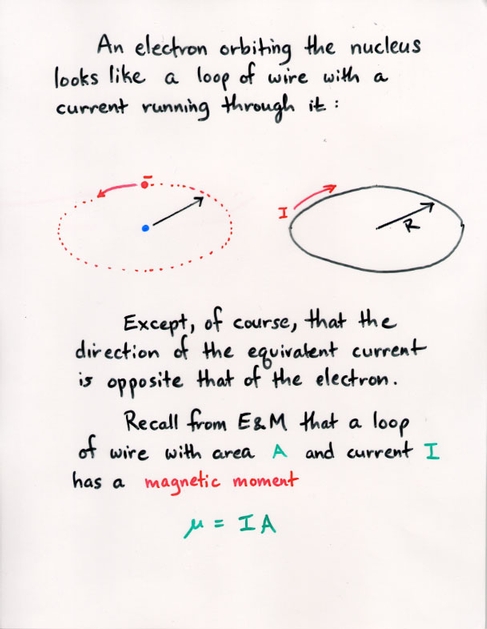
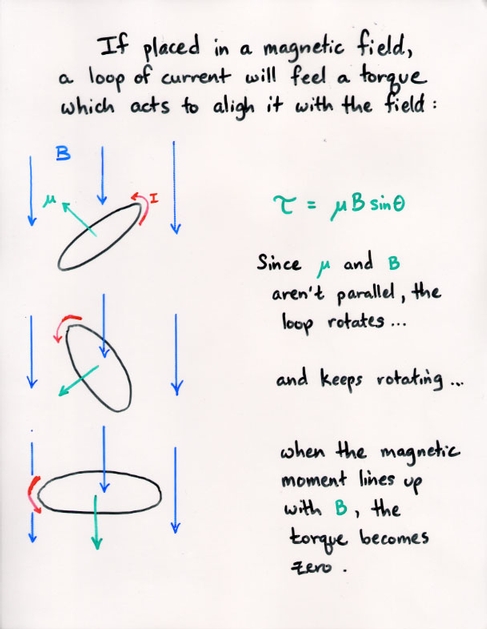
Watch the Z-component (shown on the vertical axis) of the probability of the electron's position grow as we increase the m value.


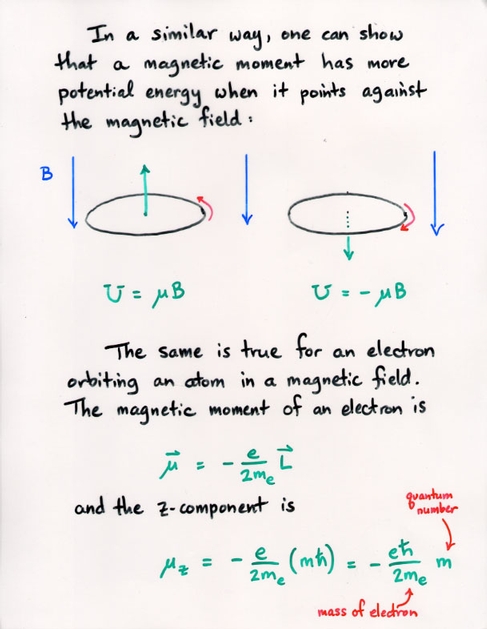
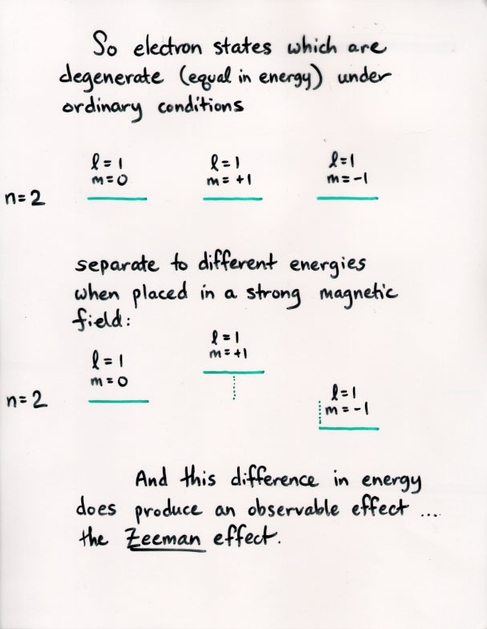
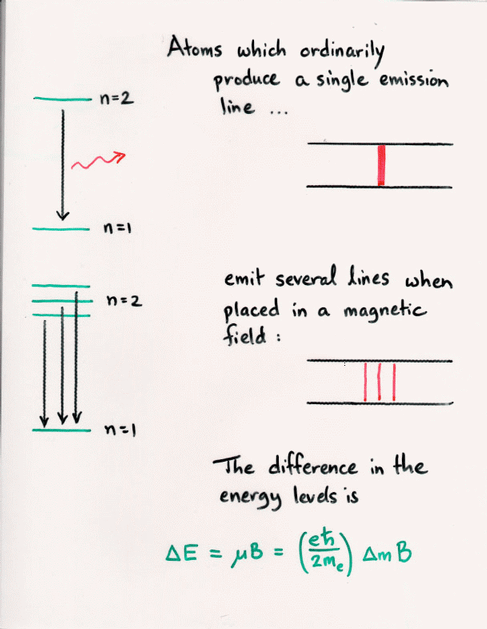
Q: If the strength of an applied magnetic field is B = 1 Tesla,
what is the difference in energy between the levels
m=0 and m=1?
Q: What is the difference in energy between the n=2 and n=1
levels of hydrogen?
Q: How big is the Zeeman effect, compared to ordinary
changes in energy levels due to jumps in n?
Just for fun: the radiation emitted by a hydrogen atom in the example above falls within the radio portion of the spectrum. Can you figure out out where -- if anywhere -- that signal would appear on an old-time radio dial?

Image courtesy of
Ralph Brandi
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.