
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.

Mendeleev's original table:
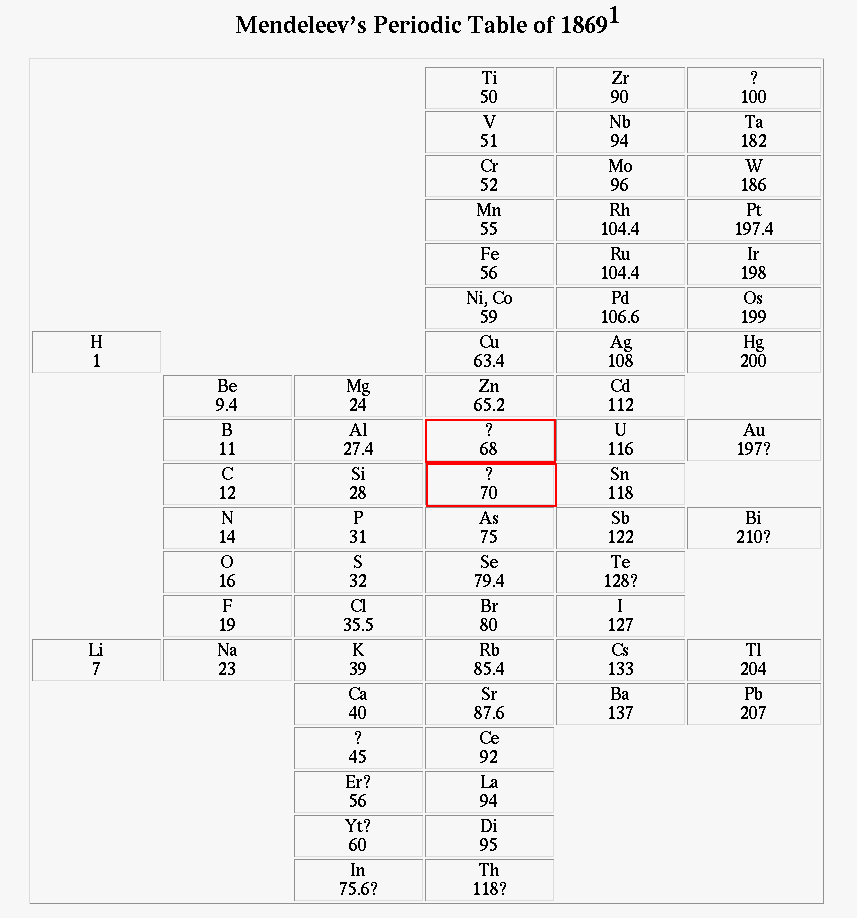
Mendeleev's table had several empty places. He asserted that new elements would be found to fill them, and -- this is important -- predicted the properties that they ought to have.
On the Relationship of the Properties of the Elements to their Atomic Weights D. Mendelejeff, Zeitscrift für Chemie 12, 405-406 (1869); translation by Carmen GiuntaOne can predict the discovery of many new elements, for example analogues of Si and Al with atomic weights of 65-75.(Mendeleev's later paper, published in 1871, went into more detail. For example, Mendeleev predicted that "eka-aluminum" would have a density of 5.9 grams per cc)
Q: His "eka-aluminum" was discovered in 1875,
just a few years after the prediction.
Its density turns out to be 5.91 grams per cc.
What do we now call it?
Q: His "eka-silicon" was discovered in 1886.
What do we now call it?
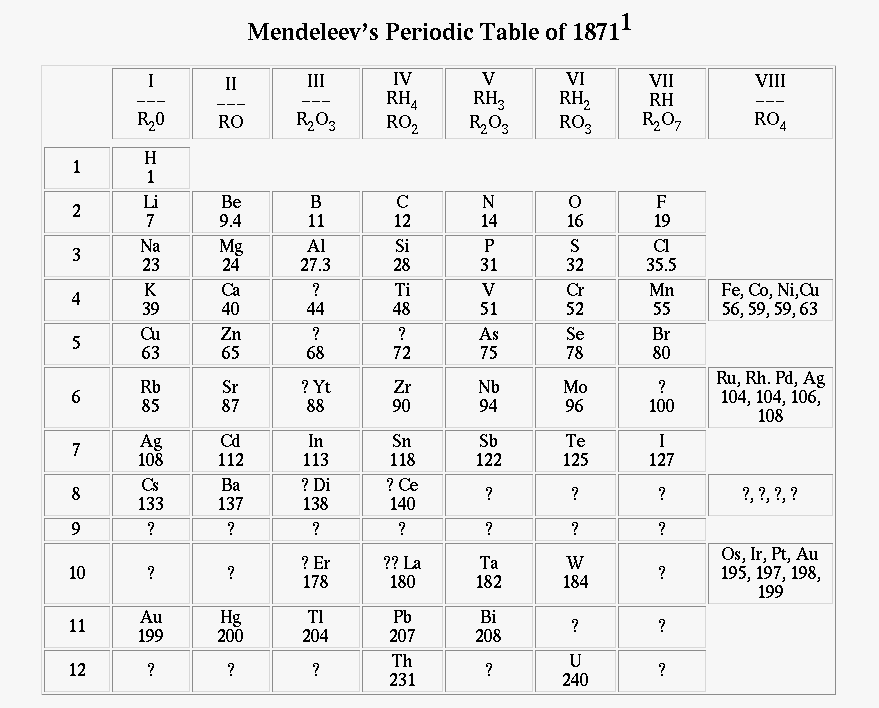
Mendeleev's improved table:

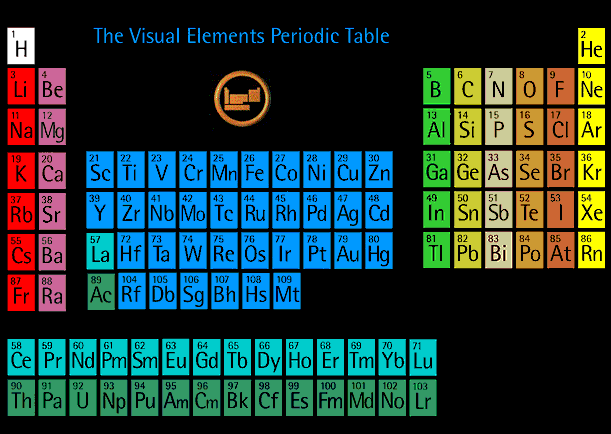
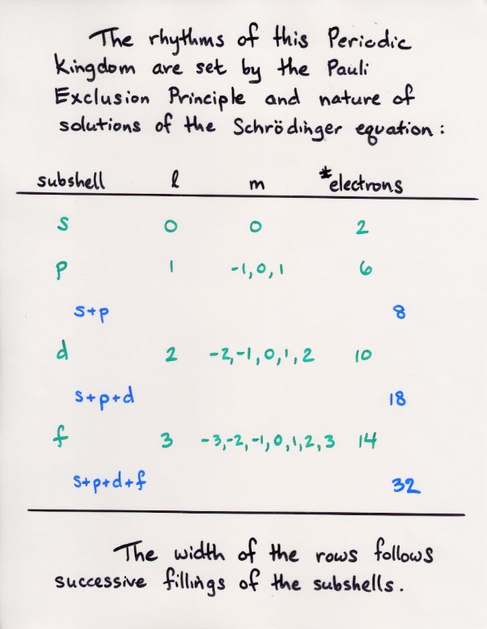

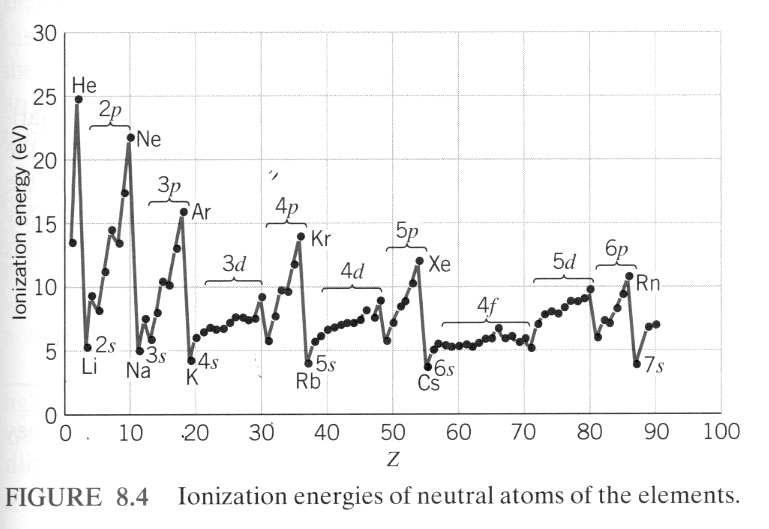
A simple line graph of ionization energy versus atomic number, from Modern Physics by Kenneth Krane (John Wiley and Sons, 1996).
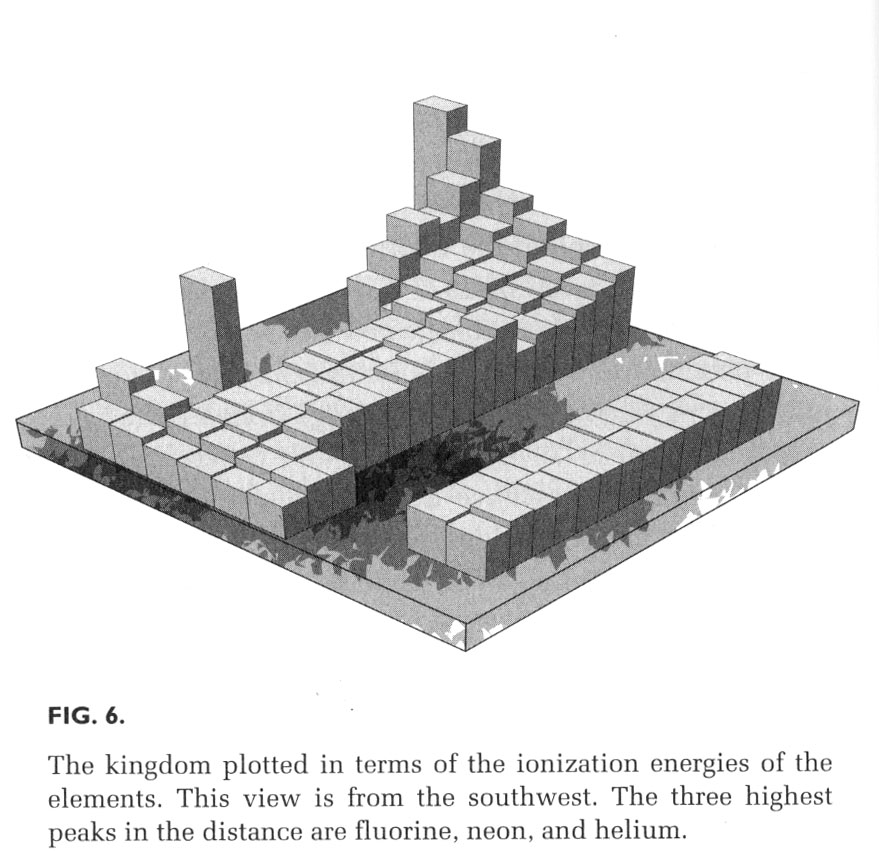
One can show the ionization energy as the altitude above the element's location on the periodic table with a 3-D graph; this one comes from The Periodic Kingdom, by P. W. Atkins (BasicBooks, 1995).
One can even create imaginary landscapes using the same idea that altitude = ionization energy, although the example below comes close to being a gratuitous use of computer graphics (it comes from the Visual Interpretation of the Table of the Elements website). Note that the point of view is identical to that of the previous example: from the southwest corner of the kingdom.

 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.