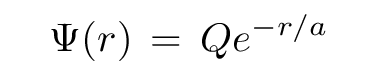
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
In our last class, we figured out that the wave function which solves Schroedinger's equation for the electron within a hydrogen atom in its ground state is
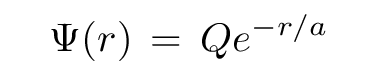
Now, since the square of a particle's wave function at some location yields the probability that the particle is at that location, you might think that we can determine the probability that the electron is within some range of radii by integrating
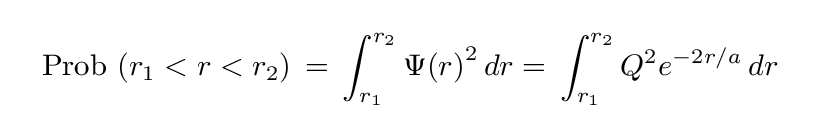
NO!
The trouble is that once we leave the familiar territory of Cartesian coordinates and venture into the Land of Spherical Coordinates, the volume element changes. Let me refresh your memory ....
Volumes in one dimension
In one dimension, things are dead simple: a single coordinate x describes everything. If we want to integrate, we must move a little extra distance in all dimensions ... which means a little extra distance dx. That's all there is.
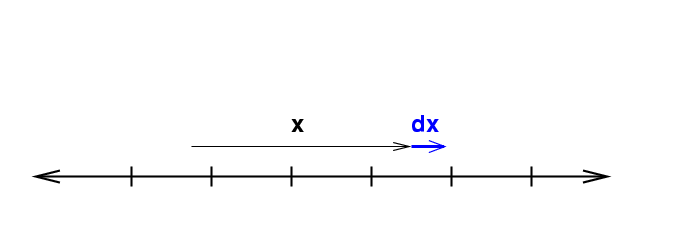
The "differential volume element" is simply
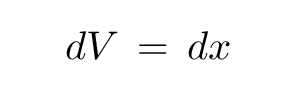
In two dimensions, thing are more interesting because there are several ways to identify a location in space. The Cartesian system uses variables x and y.
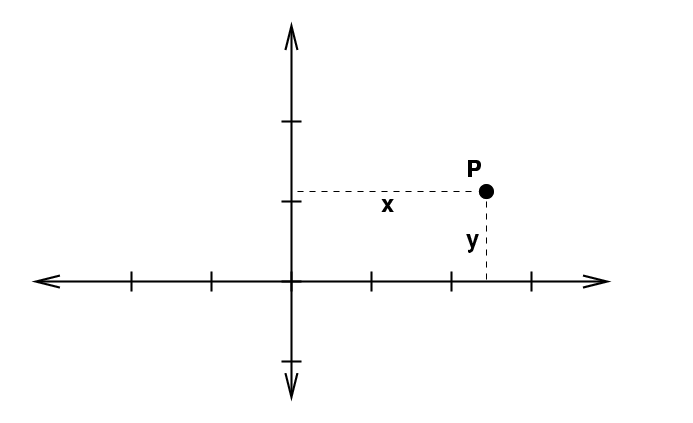
In order to integrate, we move a little extra distance in each dimension:

This sweeps out a little square box.
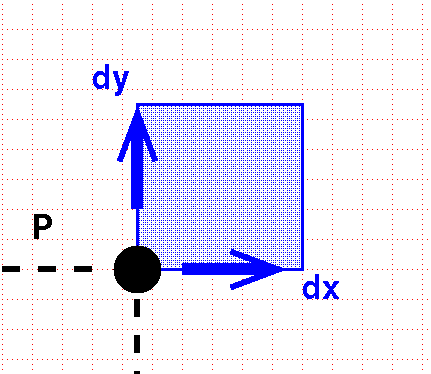
The differential volume element is the area of this box:
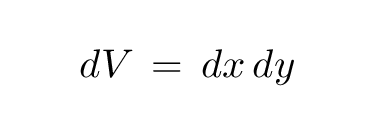
Now, we can also use polar coordinates r and θ to describe the location of a point in a plane.
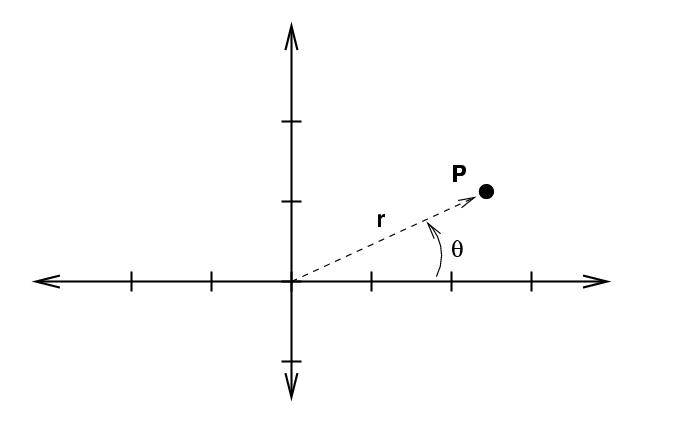
If we increase each of these variables by a teeny bit to integrate ...
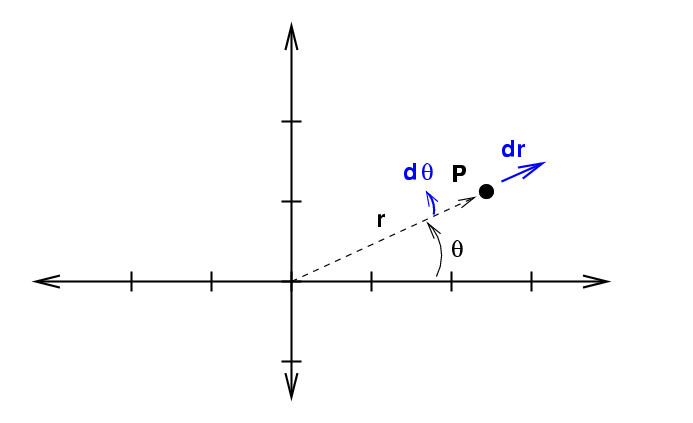
... we sweep out an area which isn't square. Instead, it has a curve along the inside and outside edges.
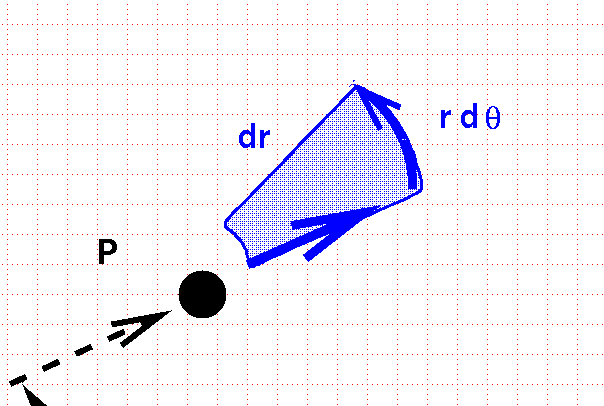
The area of this curvy box, and thus the differential volume element in polar coordinates on a plane, is
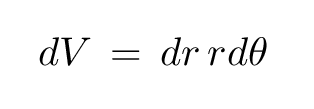
I hope you'll believe me when I say that the differential volume element in three-dimensional Cartesian coordinates is
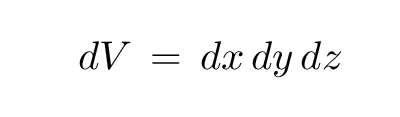
On the other hand, in three-dimensional spherical coordinates, we describe the position of an object with radius r and the angles θ and φ.

If we extend each of these three variables just a bit, we sweep out a funnel-shaped box.

In one direction, the box has straight lines of length dr. In the other directions, the edges are curved. We can compute the length of these tiny arcs using the arclength formulae in spherical coordinates, and end up with a differential volume element
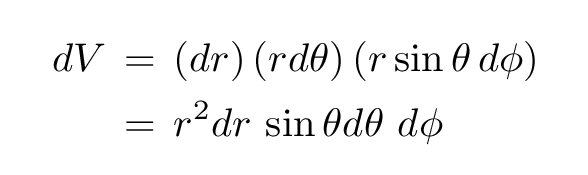
So, back to the electron within the hydrogen atom. The probability the electron is at location (r, θ, φ) within a tolerance of (dr, dθ, dφ) is

Now, if -- and that's a big if -- we can separate the wave function into three functions, each one dealing with only a single variable, then this integral becomes
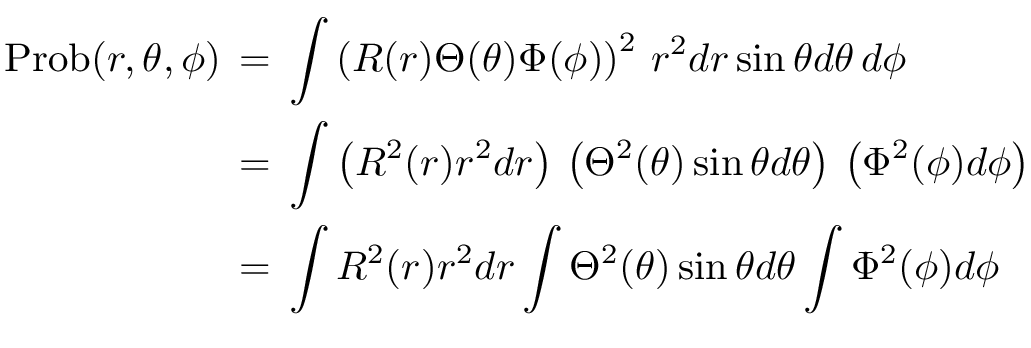
In the simplest case, corresponding to the ground state, the wave function does not depend on either angle. That means the angular functions are simply equal to 1; and that makes doing those integrals trivial.
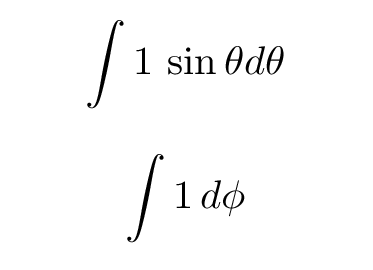
Q: What are the limits for these integrals? Q: What are the values of these integrals?
So the probability of finding the electron within some range of radii becomes
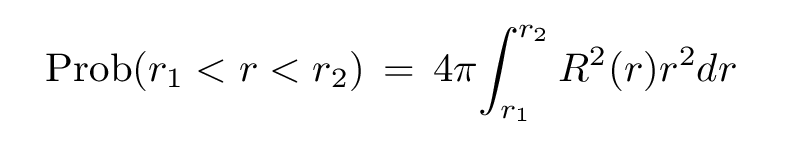
Recall that the radial function is
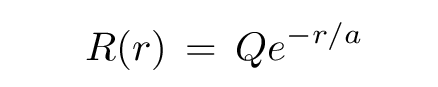
Q: Write down the integral which must be evaluated.
We can simplify this just a little bit by making a substitution: let u = 2r/a. Then we get

You can look up that integral in a table; the result isn't too ugly.
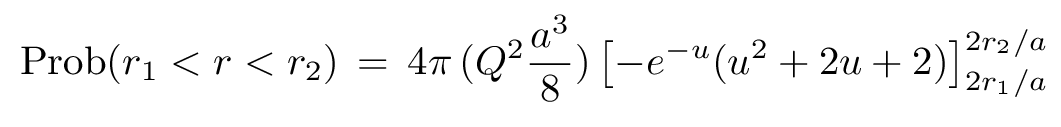
Q: If we compute the probability that
the radial position of the electron
must be between zero and infinity,
what must we get?
Q: Can you solve for the value of the
coefficient Q in our radial
wave function?
If you compare the value for Q with the one in your textbook (see section 7.2), you'll see a difference. We have placed the entire normalization condition into the coefficient Q of the radial function. The book chooses to split the normalization condition between all three functions -- the radial, polar, azimuthal. When you use the entire wave function to compute some desired quantity, both choices will yield the same result.
So, where is the electron most likely to be? We can answer this question in two ways.
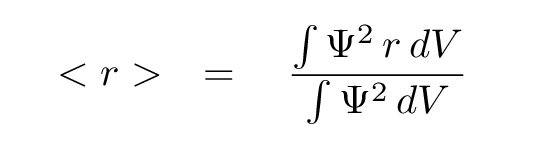

Either way, we find that the electron within a hydrogen atom in its ground state is probably going to be somewhere near the Bohr radius.
You can find more examples of wave functions and probability distributions in textbooks and on web sites. Below are some examples made using Paul Fastad's hydrogen atom applet.
In each example, the radial function is shown in the top-right panel of the figure below, labelled "Rnl(r)". The bottom-left panel provides an illustration of this function, looking down at the atom from above.
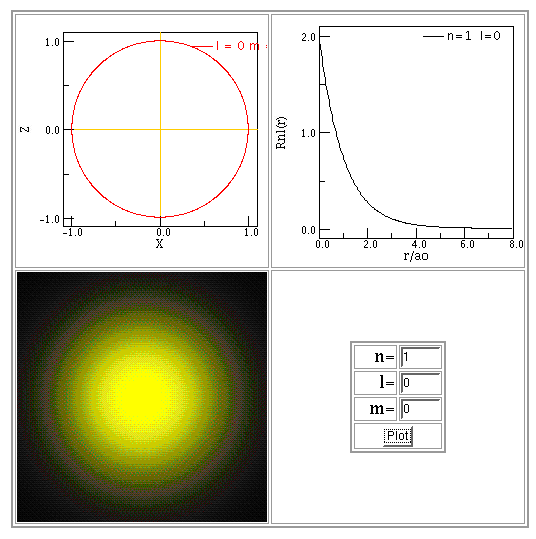


 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.