 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
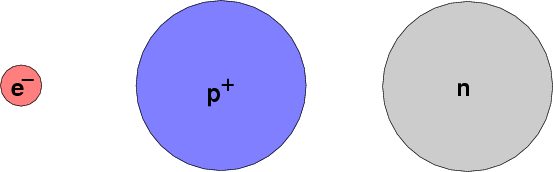
Let's meet the cast of characters who populate the world of the atoms. You may not see some of these very often in the Modern Physics class, but they will pop up if you continue to study physics, or even read the newspapers.

These three particles are often described as the "building blocks of matter." One can put them together to form any element. Although we have discovered that the proton and neutron may be composed of even smaller sub-units (quarks), it is still useful and convenient to treat them as fundamental units. The electron, as far as we can tell, is truly fundamental: it can't be broken down into any smaller pieces.
The electron and proton have opposite electric charges, and so are usually found in equal numbers. The neutron has no electric charge (hence its name). All atoms contain electrons and protons, and all but one (ordinary hydrogen) contain neutrons as well. The "light" elements usually have equal numbers of protons and neutrons, but neutrons outnumber protons in the heavier elements. One isotope of gold, for example, has 118 neutrons but only 79 protons.
Your textbook's Appendix of Atomic Data provides information on the different isotopes of all the elements. If you read it carefully, you can figure out exactly how many protons and how many neutrons are in a particular isotope. It has additional information, too: the half-life of radioactive isotopes, and the relative abundance of stable isotopes.
Q: How many protons and neutrons are
in Copper-66?
Q: How many neutrons are in the most
common form of Hafnium?
Q: What is the longest-lived isotope
of Technetium?
The electron and proton are both stable particles. The neutron, on the other hand, is unstable in isolation: it will decay into a proton and electron (and something else -- see below) with a half-life of about 886 seconds. If it is surrounded by other neutrons and protons, however, the neutron will not decay.
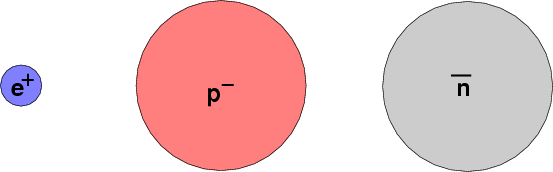
All particles have corresponding antiparticles, which have the same mass and (if applicable) size, but opposite charge. The electron's partner, the antielectron, is so common that it has a name of its own: the positron.
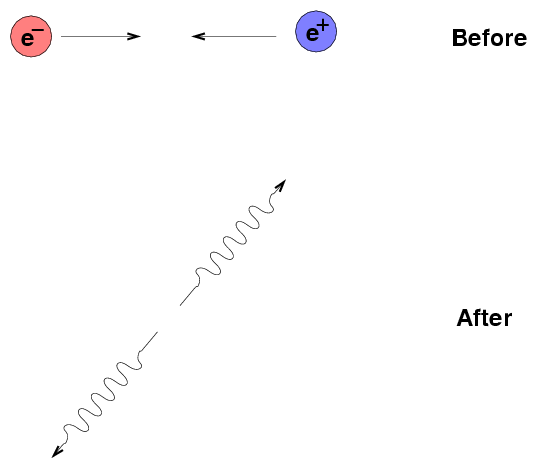
When a particle meets its antiparticle, the two annihilate each other, turning their combined rest mass into energy according to Einstein's equation
2
E = m * c
It's obvious that the positron isn't an electron, because its charge is positive, not negative. In a similar way, an antiproton is easy to distinguish from a regular proton. But how can one tell an antineutron from an ordinary one? Their electric charge is the same: zero.

In diagrams and equations, physicists often use a horizontal bar placed directly over the symbol for a particle to mean "this is the anti- version".
Near the end of the nineteenth century, scientists investigated the phenomenon of radioactivity. Some materials gave off heat and energy as they transmogrified into other materials. For example, radium emits radiation as it turns into radon, which in turn emits radiation as it becomes polonium, and so on down a chain of seven more reactions until it reaches a stable isotope of lead. It eventually became clear that there were three classes of radiation.
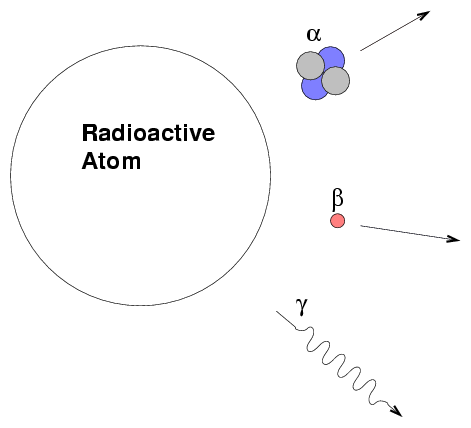
mass (kg) charge (C)
------------------------------------------------------------
alpha
------------------------------------------------------------
beta
------------------------------------------------------------
gamma
------------------------------------------------------------
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.