
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.


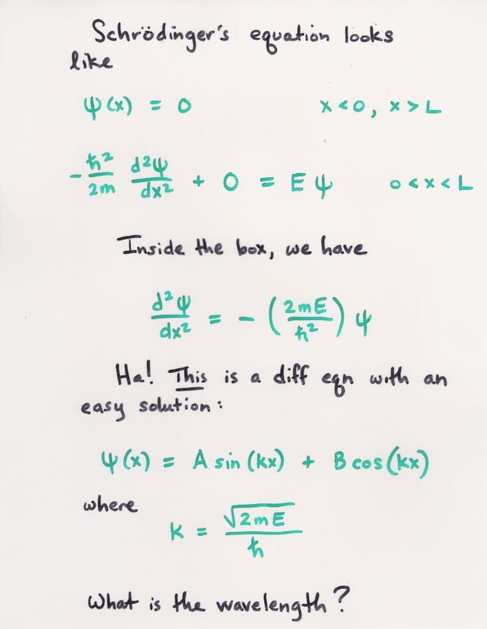
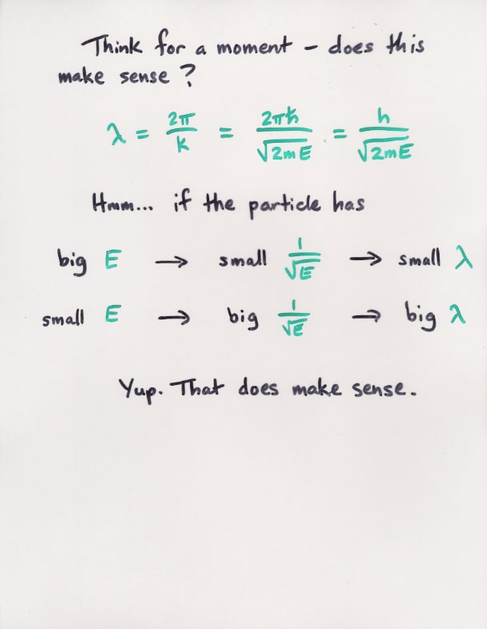
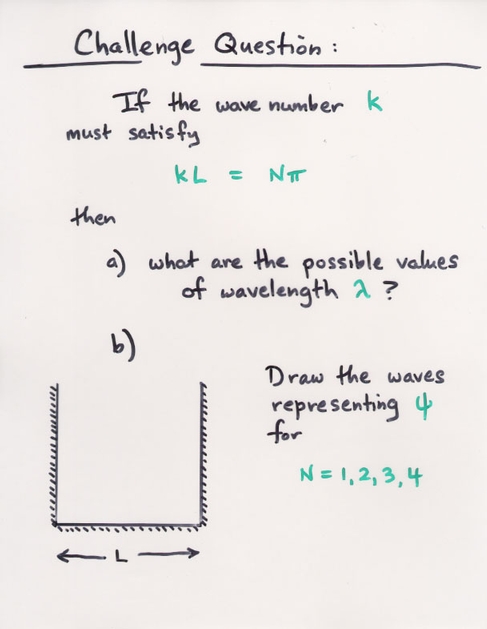

Q: You put an electron into a 1-D box
of width L = 1 Angstrom.
What is the energy of the ground
state of the electron? Express
your answer in eV.



Okay, let's use this wave function to answer a real question.
Q: When the particle is in the
ground state, n=1, what is its most
probable position?
We can answer this in two ways: one graphical, one mathematical. The graphical method involves sketching the wave function, and the square of the wave function, over the space of the box.
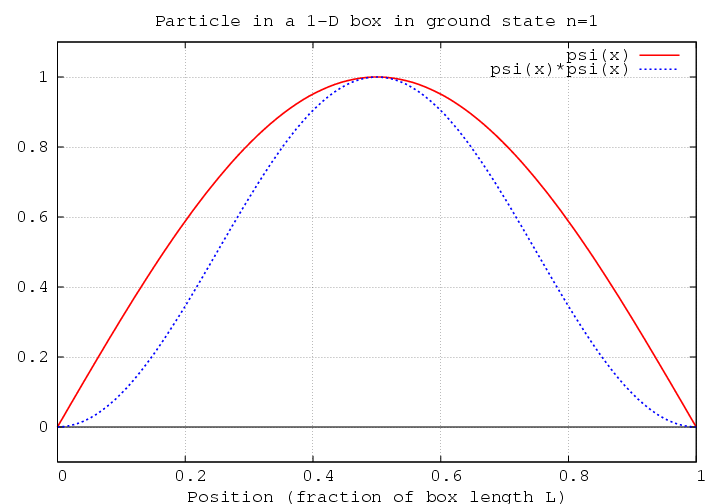
The most probable position is where the square of the wave function has the largest value.
The mathematical way of answering this question is to integrate the square of the wave function, times the quantity of interest x, over the entire box.

Q: Can you write down this integral? Q: Can you perform this integral? Q: What is the most probable value of position "x" ?
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.