 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.

This project must be done by individuals. It may help to read section 8.5 in your textbook, or sections 4.3-4.5 of Asimov's Understanding Physics: volume III.
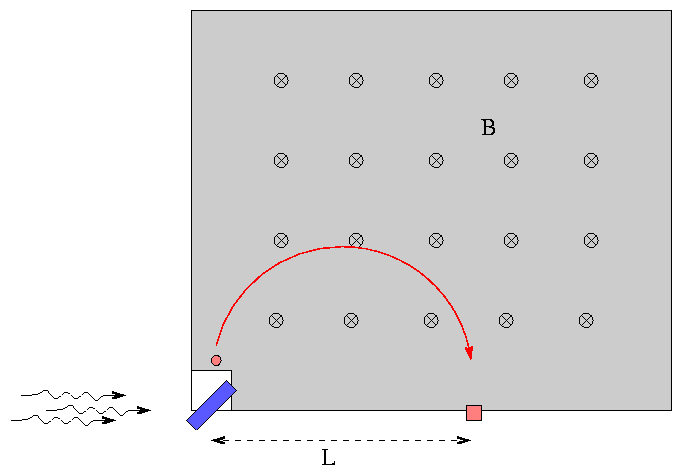
Inspired by Moseley's example, Fred decides to identify a small sample of some metal using his knowledge of K- and L-shell electron energy levels. First, he reads section 4.6 of the textbook. He sets up a box on a table with a uniform magnetic field of strength B = 1 mT pointing straight down through the table (a picture of the box from above is shown in the figure). He places the metal sample (the blue rectangle) in a hole at one corner of the box. He then shines X-rays with a precisely controlled energy of E = 25.00 keV on the metal. The X-rays knock electrons free from atoms in the sample. Some of the electrons happen to fly into the box, where the magnetic field bends their paths into a circle. The electrons eventually run into the side of the box as shown, which is lined with photographic film.
Fred shines light on the sample for several hours, the develops the film. He notices two dark spots on the film where many electrons have landed: at distances L = 15.2 cm and 99.6 cm from the sample.
What is the element in Fred's sample? Show all your work.
Hint: The equation for Moseley's Law in the text is not exact enough for this problem -- it is only an approximate fit. You need to find the precise K-shell and maybe L-shell binding energies for elements. These correspond to the K-shell and L-shell absorption edges, if that helps.
Last modified 02/19/2004 by MWR
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.