A value for the charge on the electron will be determined by measuring the mass deposited from solution in a cathode and the quantity of electric charge transferred during an electrolytic deposition.
First, to show the connection between the electrical and physical changes in a copper electrode. Second, to measure the amount of charge on a single electron.
Cutnell and Johnson 20.1. See Fig 20.5.
In this experiment a battery is connected to two electrodes which are in a copper sulfate solution (CuSO4) containing doubly positive copper ions (Cu2+) and doubly negative sulfate ions (SO42-). When a current is caused to move in the circuit, the negative ions are attracted to the anode, (positive electrode) while the positive copper ions are attracted to the cathode (negative electrode). As long as the current is maintained, copper ions will thus be deposited on the cathode where they acquire the electrons needed to become copper atoms. The increase in mass of the cathode can be measured, as can the magnitude and duration of the current. Since the deposition of one copper ion corresponds to the transfer of two electrons, it is possible to calculate the electronic charge from the data mentioned and values for Avogadro's number and the atomic mass of copper. With the apparatus provided, the results can be expected to be within 5% of the accepted value.
In this experiment, you will handle two chemicals: isopropyl alcohol and copper sulfate. Here's a small amount of information know about each one -- you can find more information in the blue folder by the sink in the lab; it hold Product Safety Data sheets for each one.
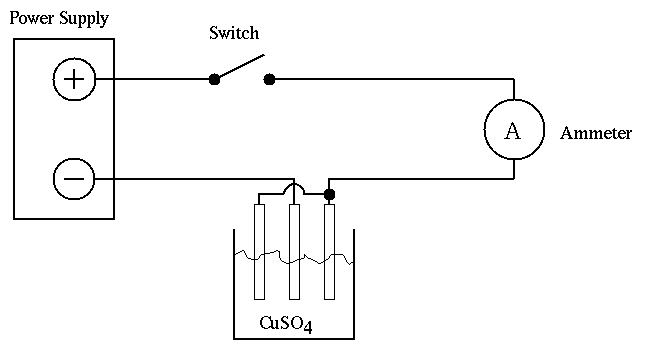
The coulometer used consists of a pair of plates connected together to form the anode and a single central plate forms the cathode (see Figure 1.). It is important that the central electrode be negatively charged. If it is positively charged, it will be found that a mass loss rather than a gain occurs (Why?).

Figure 1. Wiring Diagram for Coulometer
Before assembling the circuit, clean all the plates by a light sanding with dry emery cloth. Rinse in water and use the cleanest plate for the central position. Clamp all three plates in the coulometer cap and fill the coulometer glass with copper sulfate solution (be sure the cap clamps are not in the solution as this makes the coulometer inoperative). Now wire the circuit according to the diagram with the knife switch open. Do not turn on the power supply until your wiring is verified by the instructor.
Open the knife switch, remove the central plate being careful to HOLD IT BY THE EDGES ONLY. Dip it into isopropyl alcohol and dry it with a CAREFUL air blast. Determine the initial mass (mi) of the plate on a Mettler balance. Replace the plate in the coulometer and close the knife switch, noting AND recording the EXACT time at which the current is restored. Adjust the power supply as necessary to maintain a current of 0.50 amperes. Continue the current for a period of about 35 minutes. At the end of this period open the switch, noting AND recording the EXACT time the current stops. The time interval of deposition will be the difference in the two recorded times and should be known to an accuracy of one or two seconds. (35 minutes is NOT the same as 35 minutes and 00 seconds!)
Remove the central plate, HOLDING IT BY ITS EDGES ONLY, rinse in clean water followed by isopropyl alcohol and air dry. Do NOT dry the plate with a towel. Determine the new mass (mf) of the cathode and record it. Return the solution to the bottle, rinse and dry the coulometer glass and return all apparatus to the places where you obtained it.
During the electrolysis the total charge Q transferred (in Coulombs) is given by
Q = It Eqn 1
where I is the current (in Amperes)
and t is the deposition time
(in seconds). A second expression for the total charge comes
from the mass of deposited copper (mf - mi) and the atomic mass
of copper (M).
It should be equal to
Q = (# ions stuck on plate) * (charge per ion) Eqn 2a
It turns out that one can figure out the number of ions on the plate by comparing the mass of added copper and the mass of a single copper atom; specifically, one can write the above expression as:
N
Q = (mf - mi) --- 2e Eqn 2b
M
where N is Avogadro's number and e is the charge
on the electron.
Explain how Equation 2a turns into Equation 2b.
Draw a picture of the experiment, with microscopic closeup pictures of the action at each electrode. Label ions and electrons, and show their direction of movement.
Using Equation 1, calculate the total charge transferred during the deposition. Use this result in Equation 2b and solve for the electronic charge e. The atomic mass of Cu is M = 63.54 g/mole. Calculate the percent difference between the value and the currently accepted value of 1.6 x 10^(-19) Coulombs.
Extra Credit!
Measure the area over which the new copper was deposited, and (using a vernier caliper) the thickness of the new layer. Assuming the copper atoms are little cubes, packed tightly together, how large is each atom?
Last modified Dec 13, 2001 by MWR