 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Of what is the universe made? What are the ingredients for the Cosmic Recipe? If we can answer these questions, we may gain some clue to the history of our universe.
People have long known that the stars are far, far away; in the nineteeth century, astronomers finally measured the distances to a few nearby stars with reasonable accuracy. The results were so large -- thousand of trillions of miles -- that most people figured we'd never be able to visit them or learn much about them. After all, we can't go to a star, grab a sample, and bring it back to earth; all we can do is look at light from the star. In fact, at least one prominent philosopher and scientist went on the record as saying that we'd never be able to figure out their compositions.
Of all objects, the planets are those which appear to us under the least varied aspect. We see how we may determine their forms, their distances, their bulk, and their motions, but we can never known anything of their chemical or mineralogical structure; and, much less, that of organized beings living on their surface ...(Comte refers to the planets in the quotation above; he believed that we could learn even less about the stars)Auguste Comte, The Positive Philosophy, Book II, Chapter 1 (1842)
But just a few decades after this pessimistic statement, astronomers were starting to identify elements in the solar atmosphere. We now have a good idea about the chemical makeup not only of the stars, but of the entire visible universe...
It's easy to figure out the chemical composition of the Earth: just dig up some dirt, and analyze it. Well, maybe it's a bit more complicated than that.
We live on the surface of the Earth, which may contain a different mix of elements than the inner regions. Up here, at the surface, we can divide the environment into several pieces:
If we count the total number of atoms in each component, the atmosphere is by far the least important, and the solid crust by far the most important. One could pretty much ignore the air and the water...
But, of course, even the solid crust is just the outer layer of a very much larger interior. We can't dig down more than a few miles into the Earth, so we can't determine the composition of the interior directly; but we can find clues in the material ejected from volcanos, and also in the behavior of seismic waves as they move through the Earth. Since we know the radius and the mass of the Earth, we can calculate its overall average density, which turns out to be about 5500 kilograms per cubic meter, or about 5.5 grams per cubic centimeter: 5.5 times denser than water. The upshot of all this indirect evidence is that we believe the interior of the Earth is made up of
Overall, since the core and the mantle comprise most of the atoms of the Earth, the chemical composition of our planet is dominated by iron, oxygen, and silicon.
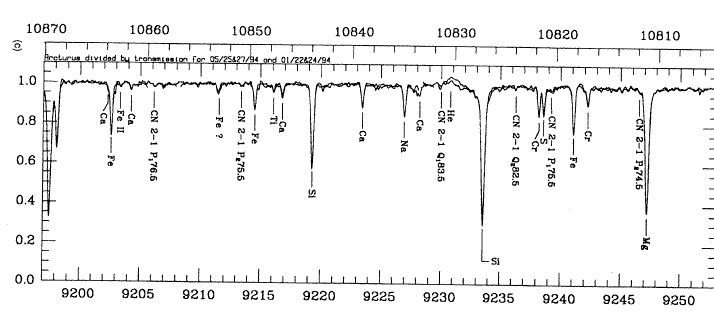
In the early days of astrophysics, scientists thought that the stars were probably similar to the Earth in chemical composition. When they passed starlight through a prism and examined the resulting spectrum, they found absorption (and occasionally emission) lines of many elements common here on Earth. For example, here's a portion of the spectrum of Arcturus (taken from a paper by Hinkle, Wallace and Livingstone, PASP 107, 1042, 1995):

Now, different stars have spectra which look very different (click on image to see larger version):
Does this mean that the chemical composition of stars varies wildly? Initially, scientists thought the answer was "yes."
In the nineteen-twenties, Cecilia Payne studied the spectra of stars, and devised a way to figure out the temperature and true chemical composition of stars. She concluded that the atmospheres of stars were
Our galaxy contains not only stars, but also clouds of gas and dust. Some glow brightly, lit up by nearby stars:
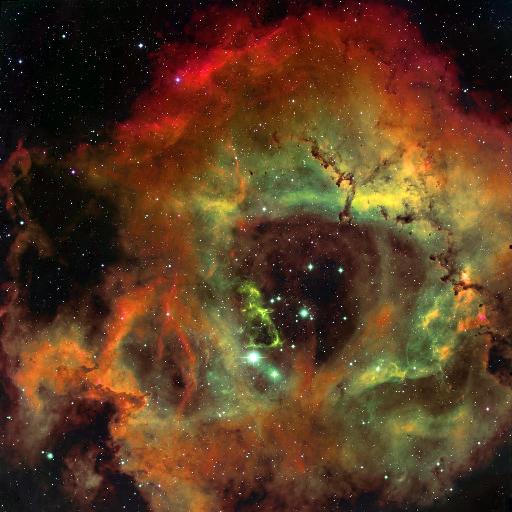
Other clouds appear dark, because they absorb and scatter the light which tries to pass through them:

It is often easier to determine the composition of nebulae than of stars, since we can see into the center of the nebula. The spectra of these objects show that they, too, are almost completely made of hydrogen and helium, with tiny amount of other elements.
When we look at different galaxies, we find some variation in the amount of heavy elements. The Milky Way, for example, has more iron (relative to hydrogen) than the Large Magellanic Cloud; and the Large Magellanic Cloud has more iron (relative to hydrogen) than the Small Magellanic Cloud.
We believe that heavy elements can be created by the fusion of light elements at the centers of stars.
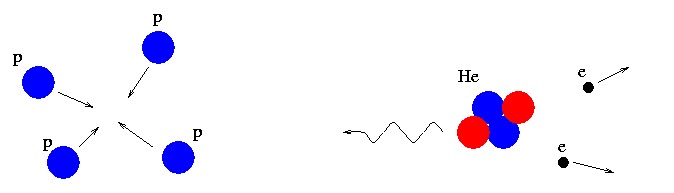
Galaxies with lots of heavy elements must have had several generations of stars, some of which have ejected material from their interiors into the interstellar medium and enriched it with helium and heavy elements.
Astronomers use the letters X, Y and Z to denote the fraction of material (by mass) which made up by hydrogen, helium, and everything else:
X = 1.0 Y = 0.0 Z = 0.0 pure hydrogen
X = 0.5 Y = 0.5 Z = 0.0 hydrogen/helium mix
X = 0.0 Y = 0.5 Z = 0.5 helium/heavy mix
When we analyze the composition of nebulae in different galaxies, we find a slight correlation between the fraction of helium and the fraction of heavy elements:
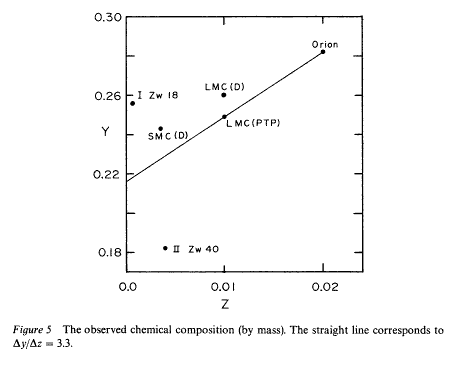
Why should there be such a connection? We think the answer is
If we can find galaxies which have had little star formation since they were formed, we can use them to measure the primordial abundance of helium, relative to hydrogen.
number of hydrogen atoms
------------------------ = 12.5
number of helium atoms
In our own corner of the Milky Way, this ratio is currently about 10. There has evidently been quite a bit of nuclear processing of hydrogen into helium by previous generations of stars in our galaxy.
But we are left with two big questions:
There's another question which might arise, too:
For more information, see
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.