 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
We know that life evolved on Earth. But did it also form on other planets, either in our Solar System or elsewhere? At the moment, we just don't know. But we don't have to let that ignorance stop us from speculating on some of the factors involved in the formation of life.
In the following discussion, I consider "life" to mean only life as we know it. There could very well be alien beings made of magnetic field loops or ionized hydrogen, but we couldn't really engage in any quantitative discussions about them.
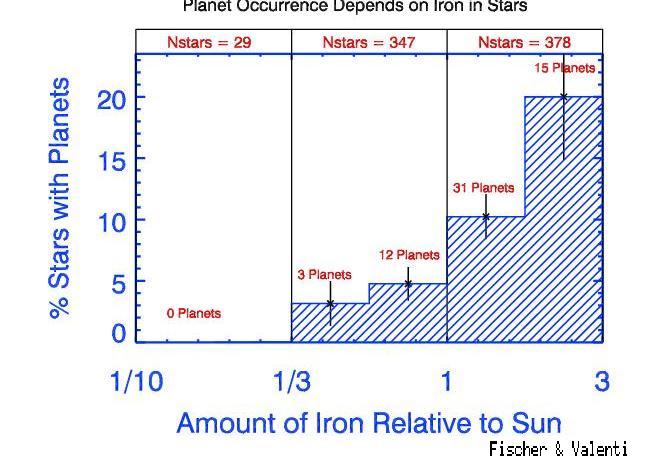
In recent years, astronomers have started to find quite a few planets around other stars, using several different techniques. The radial-velocity technique has been the most successful in the past few years. What fraction of the stars examined (*) by planet hunters turn out to have planets (**)?
So you might conclude that between one-tenth and one-fifth of all stars might have planets ...
The fine print:
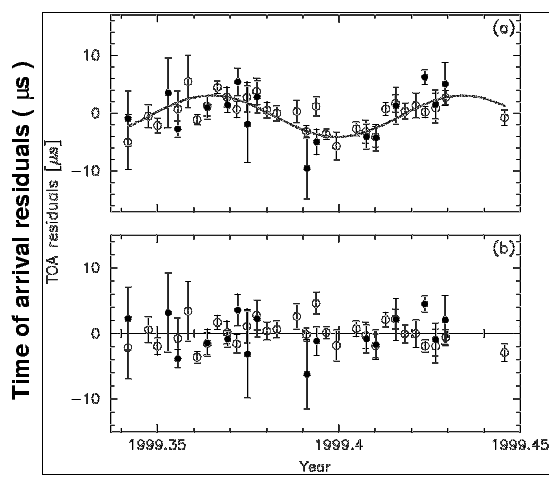
Actually, there is one case in which we have detected planets with masses similar to the Earth orbiting around some other star. About a decade ago, as radio astronomer Alexander Wolszczan studied a pulsar, he noticed a peculiar periodic shift in the arrival times of its pulses.
These measurements were MUCH more precise than one could do with ordinary spectrographs and ordinary stars. Note the units of time on the graph below.
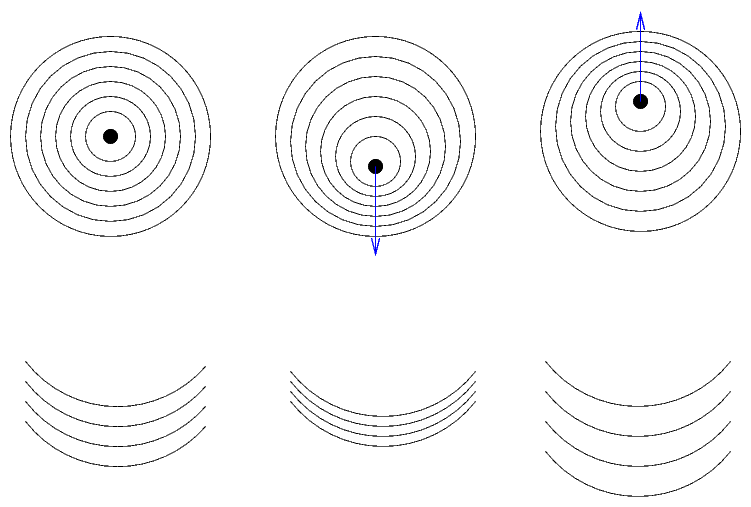
Planets of roughly Earth mass! Hooray! Or so one might think at first. But look closely at the system:

These planets are very close to a pulsar...
Q: What sort of star was originally at the center of this
system, before it turned into a pulsar?
Q: How did it turn into a pulsar?
Hmmm. Maybe these aren't the best planets for life. Can we do any better?
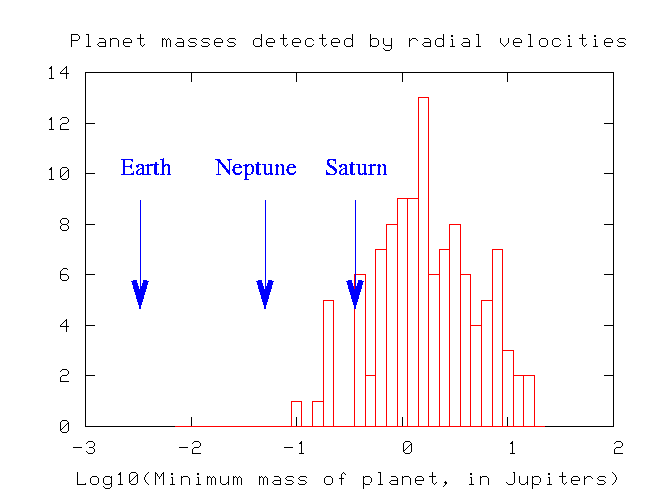
Let's take a second look at what we know about planets orbiting other stars. Most are detected by their gravitational influence on their host star:
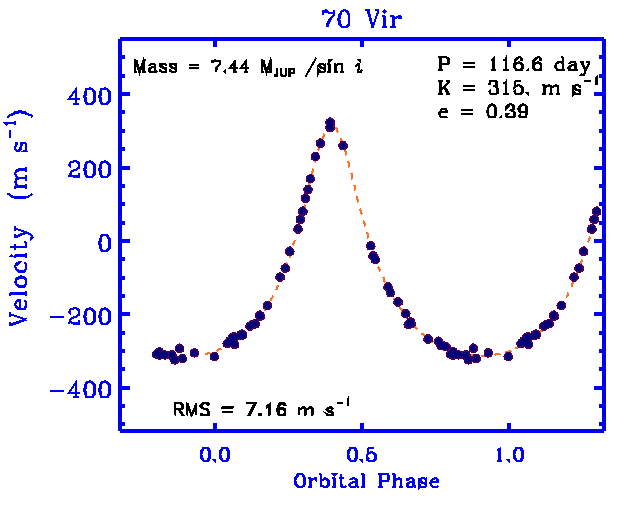
That means that all we really know is
We find that almost all these planets have masses much, much larger than the Earth. Is that important?
Yes, we think so. Take a look at the planets in our own solar system. They break up into three groups:
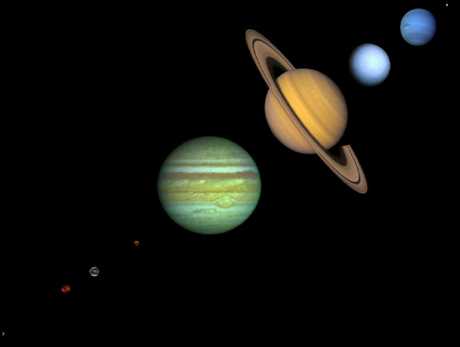
The only ones which we humans could easily inhabit are the terrestrial planets
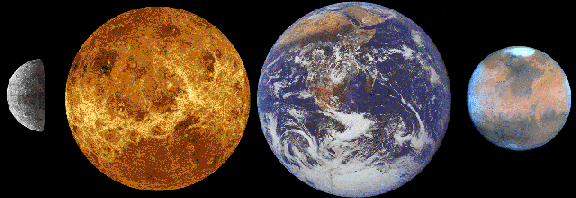
because they have solid surfaces and surface gravities which wouldn't break our spindly little legs.
So, we know the MASS of planets around other stars --- but do we know anything about their SIZES? It turns out that in just a very few cases, we do. The transit technique tells us how large a planet is, relative to its star. Since we have a decent guess at the size of most stars, we can figure out the size of the planet. The Extrasolar Planets Encyclopaedia currently (as of Nov 10, 2004) lists 6 planets with measured sizes:
name mass (Jupiters) radius (Jupiters)
---------------------------------------------------------------
OGLE-TR-56 b 1.45 1.23
OGLE-TR-113 b 1.35 1.08
OGLE-TR-132 b 1.19 1.13
TrES-1 0.75 1.08
HD 209458 b 0.69 1.43
OGLE-TR-111 b 0.53 1.0
----------------------------------------------------------------
So all these planets have roughly the mass of Jupiter, and roughly the size of Jupiter. That combination yields a surface gravity of about 2.5 times the Earth's: in other words, if you were standing on one of these planets (or on an airship floating in the atmosphere of one), you would weigh about 2.5 times what you weigh here on Earth. That's not good news for us humans, but it probably wouldn't bother some creature which had evolved there.
Here on Earth, liquid water is necessary for life to thrive. Therefore, if we are looking for Earth-like organisms, we need to discard any planets which are too hot or too cold for liquid water.
The temperature of a planet depends largely on two factors:
In real life, there are complications -- the effects of albedo, radiation transfer through the atmosphere, internal generation of energy -- but to a very rough approximation, we can calculate the temperature of a planet in the following way:
[ L ] 1/4
Temp of planet = [ ----------------- ]
[ 16 * pi * s * R^2 ]
where
L = luminosity of the star (Watts)
s = Stefan-Boltzmann constant
= 5.67 x 10^(-8)
R = radius of planet's orbit (meters)
Does this really work? If we plug in the values for the Sun and the Earth's orbit, we find a temperature of 278 Kelvin = 5 degrees C = 41 degrees F. That's roughly right. Earth's actual temperature is a bit warmer because our atmosphere traps some of the heat which would be radiated away into space.
Okay, so what about all those other planets discovered around other stars? Recall that a lot of them are pretty close to their stars...
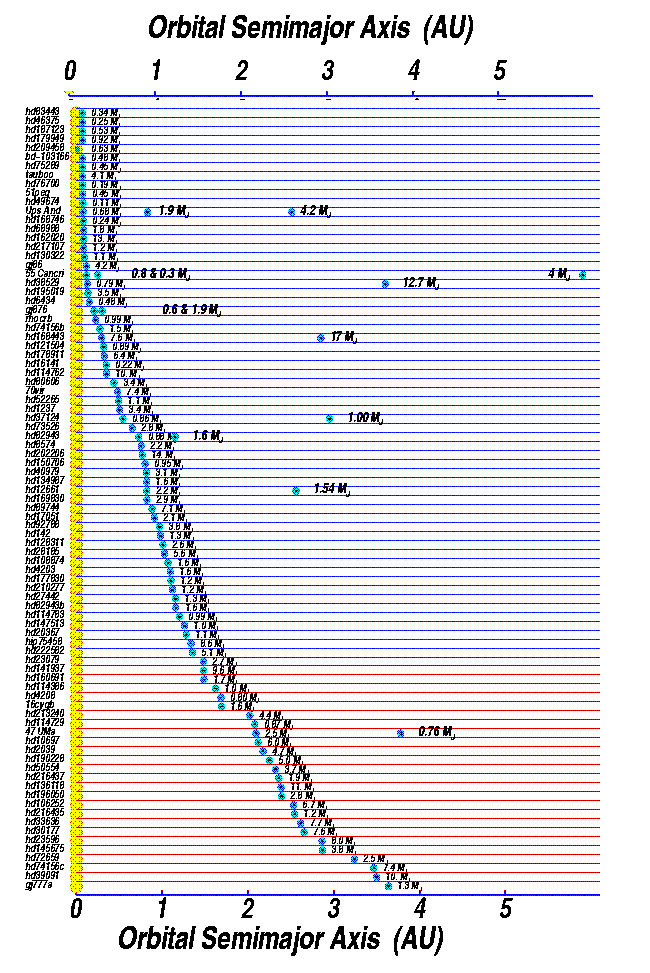
That means that they will be pretty hot. Consider, for example, the very first planet to be discovered by radial-velocity measurements back in 1995, the one which orbits star 51 Peg:
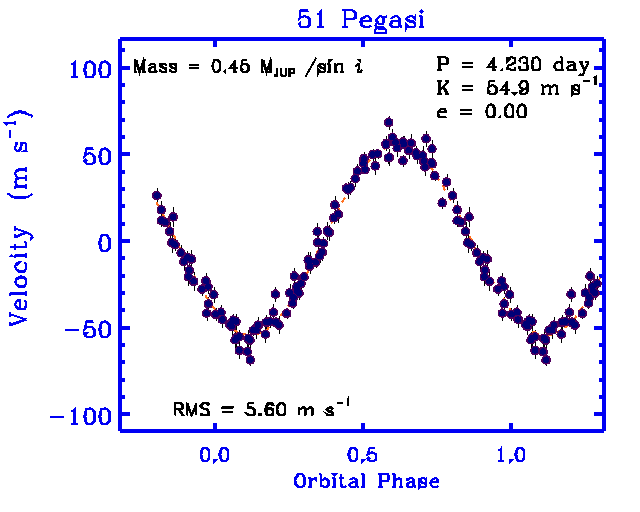
According to the California & Carnegie Planet Search, this system has
Q: Estimate the temperature of the planet
circling 51 Peg.
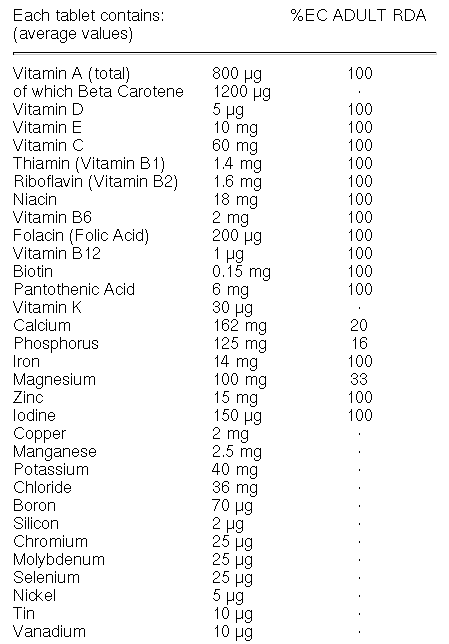
What are the ingredients needed to make a human?
lots of
plus a bit of
Here, for example, is the list of ingredients in one particular multivitamin pill.
Fortunately, most of these are pretty common, as elements go in the universe:
Element Symbol Atomic Number Number of Atoms
per million of hydrogen
-----------------------------------------------------------
Hydrogen H 1 1,000,000
Helium He 2 68,000
Carbon C 6 420
Nitrogen N 7 87
Oxygen O 8 690
Neon Ne 10 98
Sodium Na 11 2
Magnesium Mg 12 40
Aluminum Al 13 3
Silicon Si 14 38
Sulfur S 16 19
Calcium Ca 20 2
Iron Fe 26 34
Nickel Ni 28 2
-----------------------------------------------------------
Of course, it's not just the amount of each element which is important; it must also have the appropriate chemical form. Our bodies contain complicated organic molecules, consisting of tens or hundreds or thousands of atoms all joined together in very particular ways. That isn't very likely .... or is it?
In 1953, Stanley Miller and Harold Urey, at the University of Chicago, performed a simple experiment to see what would happen to simple chemicals in the atmosphere of the early Earth. They combined in a beaker gases which they believed to make up the bulk of the young Earth's atmosphere
More recent research indicates that the early atmosphere of the Earth may not have contained exactly this combination of gases. However, the Earth is a heck of a lot bigger than a beaker, and it also cooked its ingredients for a lot longer than a single week. It does seem likely that simple molecules do turn into more complicated ones if given enough time and a bit of energy.
The real proof is in the pudding: when astronomers look carefully, they find complex molecules in several forms out in space.
vinyl alcohol
glycoaldehyde

Okay, so you find a planet which is
How long will it take for this "soup" to give rise to some sort of life?
Let's look at the example of the Earth. The planet formed roughly 4.6 billion years ago. Although the fossil record isn't a perfect record, and grows more and more fragmentary the further back one looks, we have some notion of the evolution of life on the planet. Suppose that we compress the entire history of the Earth into a single calendar year ...
So, on the Earth, it took over one billion years for life to form and reach the point that it left recognizable fossils. There was then a very long period when, as far as we can tell, not much happened: the only living things were simple single-celled organisms, similar to bacteria. Roughly another THREE BILLION YEARS passed before we detect the remains of creatures as complex as a jellyfish. That's a long time with -- from our anthropocentric point of view -- not much to show for it.
It's so long a time, in fact, that it exceeds the lifetime of many stars. Remember that massive stars run through their fuel rapidly: to a rough approximation,
-2.5
lifetime ( mass )
--------------- = ( ------------ )
solar lifetime ( solar mass )
Q: The Sun will fuse hydrogen for about 10 billion
years. How long will a star of 1.5 solar masses
last? A star of 2 solar masses? A star of 3
solar masses?
This adds another requirement to the recipe for (complex) life to evolve: it must exist on a planet orbiting a relatively low-mass star. Fortunately, most stars in our Milky Way Galaxy are less massive than the Sun, so this may not disqualify too many systems.
Gee, there are a lot of factors which go into the formation of life. And we haven't even started to worry about additional factors which are important for confirming our guesses. Since we can't (yet) go off and have a look at planets around other stars, the only ways we could detect life on other planets are
The second option is worth trying ... but it places some additional constraints on our calculations:
In the nineteen-sixties, radio astronomer Frank Drake tried to combine all the factors into a single equation which would calculate the number of alien civilizations in our galaxy with which we could communicate. Here's the version of his famous Drake Equation you can find in your textbook (there are many variations):
N = N* fp nlz fl fi fS
where
N* = number of stars in the Milky Way Galaxy
fp = fraction of stars with planets
nlz = number of planets per star which lie in the "life zone"
for a long time
fl = fraction of suitable planets upon which life evolves
fi = fraction of life which become intelligent
fS = fraction of star's life for which intelligent life continues
N = number of communicative civilizations in our galaxy
We have some reasonable order-of-magnitude estimates for the first three parameters in this equation, but nothing better than wild-eyed guesses for the final three values. That means, unfortunately, that what you get out of this equation is really what you choose to put into it ...
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.