 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
We are mostly water, with bits of carbon, nitrogen, calcium, and other impurities mixed in. But we play with all sorts of materials: gasoline, plastics, gold, silicon, even (when we don't like someone) plutonium. Whence came it all?
The short answer is "deep inside a supernova", but that's not the whole story. Let's go way, way, way back to the Big Bang, and see the origin of the chemical elements.
Sherman, set the Wayback Machine for roughly fifteen billion years ago ...
The Big Bang is a name for a model of the universe which begins with a, well, a big bang. The name was coined by a scientist (Fred Hoyle) who didn't like the theory and tried to make it sound silly. Unfortunately for him, both the name and the model have become very popular.
The Big Bang is built upon three main tenets:
One of the primary successes of the Big Bang theory is its explanation for the chemical composition of the universe. Recall that the universe is mostly hydrogen and helium, with very small amounts of heavier elements. How does this relate to the Big Bang?
Well, a long time ago, the universe was hot and dense. When the temperature is high enough (a few thousand degrees), atoms lose all their electrons; we call this state of matter, a mix of nuclei and electrons, a fully-ionized plasma. If the temperature is even higher (millions of degrees), then the nuclei break up into fundamental particles, and one is left with a "soup" of fundamental particles:
Now, if the "soup" is very dense, then these particles will collide with each other frequently. Occasionally, groups of protons and neutrons will stick together to form nuclei of light elements ... but under extremely high pressure and temperature, the nuclei are broken up by subsequent collisions. The Big Bang theory postulates that the entire universe was so hot at one time that it was filled with this proton-neutron-electron "soup."
But the Big Bang theory then states that, as the universe expanded, both the density and temperature dropped. As the temperature and density fell, collisions between particles became less violent, and less frequent. There was a brief "window of opportunity" when protons and neutrons could collide hard enough to stick together and form light nuclei, yet not suffer so many subsequent collisions that the nuclei would be destroyed. This "window" appeared about three minutes after the starting point, and lasted for a bit less than a minute.
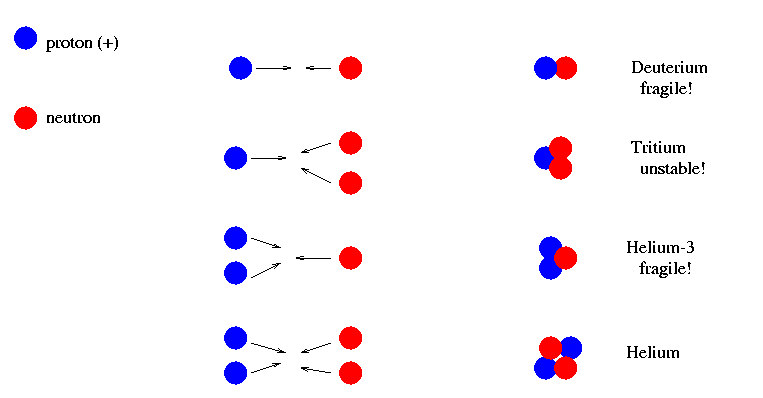
Which nuclei would form under these conditions? Experiments with particle colliders have shown us that most of the possible nuclei are unstable, meaning they break up all by themselves, or fragile, meaning they are easily broken by collisions.

Helium (the ordinary sort, with 2 protons and 2 neutrons) is by far the most stable and robust compound nucleus. Deuterium (one proton and one neutron) is easily destroyed, and so is helium-3 (2 protons, one neutron).
So, it seems that this period of hot, dense plasma would create a lot of helium. Could it create other, heavier elements, too?
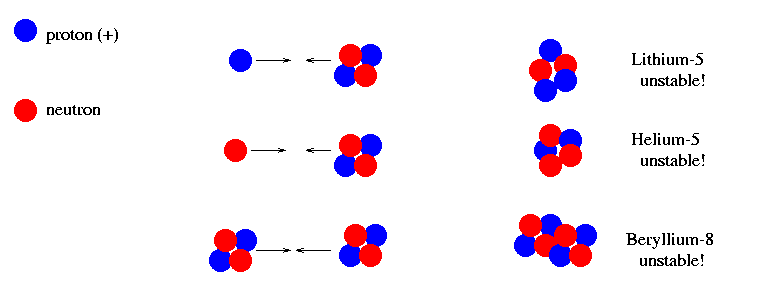
It turns out that none of the heavier nuclei which are easily made by collisions of single particles with helium nuclei, or helium nuclei with each other, are stable or robust. Almost all nuclei heavier than helium are likely to be destroyed by subsequent collisions. The only heavier nucleus which might possibly survive is lithium-7 (3 protons and 4 neutrons), but it requires the collision of a helium nucleus plus 2 or 3 other particles simultaneously, which isn't very likely.
Detailed models of Big Bang nucleosynthesis predict that the brief "window of opportunity" lasted only a minute or two. After that, about three and a half minutes after the starting point, the temperature and density dropped so much that collisions between particles were rare, and of such low energy that the electric forces of repulsion between positively-charged nuclei prevented fusion. The result is
The relative amounts of hydrogen, helium, deuterium and lithium depend very sensitively on the exact density of matter in the universe during this window of opportunity. We can use current values of deuterium, in particular, to place limits on the density of the universe during the very early stages ... which, in turn, have profound implications for the distant future.
Okay, so we have a universe full of hydrogen, helium, and nothing else. What does it do? The details are not understood, but within a billion years or so, a lot of the gas collects into giant clouds which collapse to form proto-galaxies (maybe), globular clusters, and, eventually, bona fide galaxies. When we look as far back into time as we can, we see objects that are typically somewhat smaller, somewhat bluer, and much less organized that present-day galaxies:
Now, it's true that we can't see much detail in these distant galaxies -- both because they are so far away and because they are so faint -- but what we can see suggests that the first wave of objects to collapse were messy little things, PERHAPS the building blocks of the galaxies we see today.
Within each of these giant collapsing clouds, many smaller, star-size clouds form and collapse to form stars. We think that this first round of stars, called Population III by theorists, may have been on average more massive than stars which form today. Models of stellar interiors show that a pure mixture of hydrogen and helium doesn't do a good job of blocking high-energy light from the interior of a star. The combination of (perhaps) high mass and (very probably) low stellar opacity adds up to a generation of stars which were hotter, bluer and more luminous (on average) than our contemporaries.
Spectroscopy of distant (and therefore young) galaxies reveals that many of them are very blue and contain lots of high-mass stars. It fits.
Of course, without any heavier elements in the proto-stellar clouds, no terrestrial planets could have formed around these stars. Gas giants like Jupiter, maybe; but definitely no Earth-like planets.
A nice place to visit, but I wouldn't want to live there.
Some of the first generation of stars were massive enough to explode as supernovae at the end of their lives. The heavy elements which built up inside their cores:
When these contaminated clouds collapsed, they formed Population II stars: still overwhelmingly hydrogen and helium, but with little tiny traces of heavier elements even at the start. There aren't many of these very early stars left today, but recent observations by astronomers in Europe show that one can still find a few holdouts from this very early period:
We measure the "metal" content of stars by comparing the abundance of their heavy elements to that of hydrogen. The star HE 0107-5240 has only about 1/200,000 of the iron content of the Sun. That's a tiny fraction ... but still enough to show that it cannot have been one of the very first generation of stars to form.
As time went by, generation after generation of massive stars was born, exploded as supernovae, and enriched the interstellar medium more and more in heavy elements. We check verify that this actually happened by measuring the abundance of heavy elements such as iron in galaxies at a range of distances and hence ages.
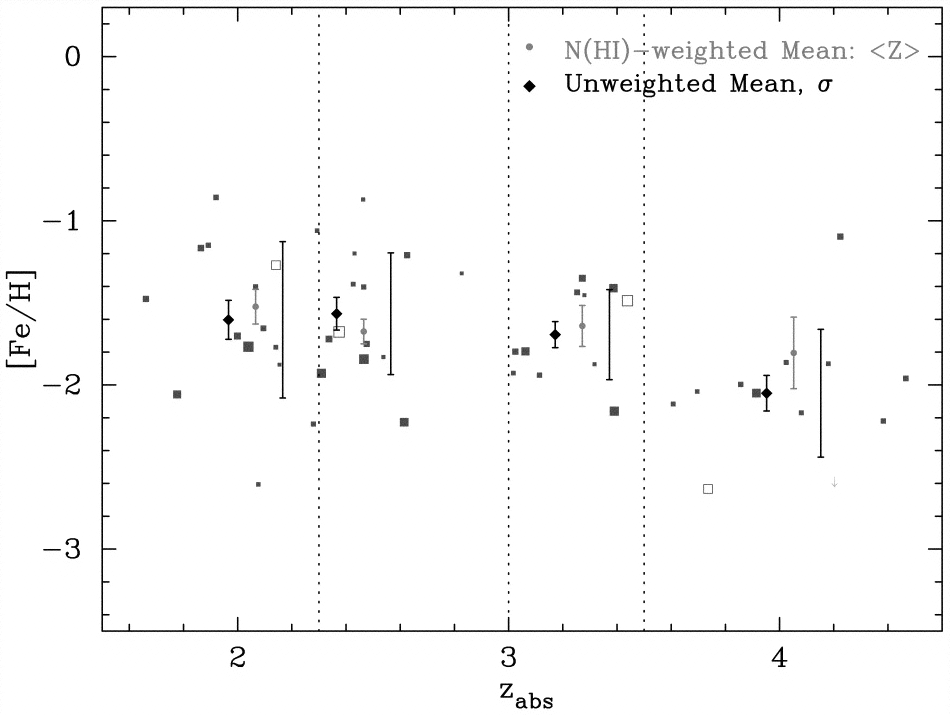
See The UCSD HIRES/Keck I Damped Ly Abundance Database. II. The Implications for details about the figure.
Here's another set of measurements from different objects, both galaxies and stars within the Milky Way:
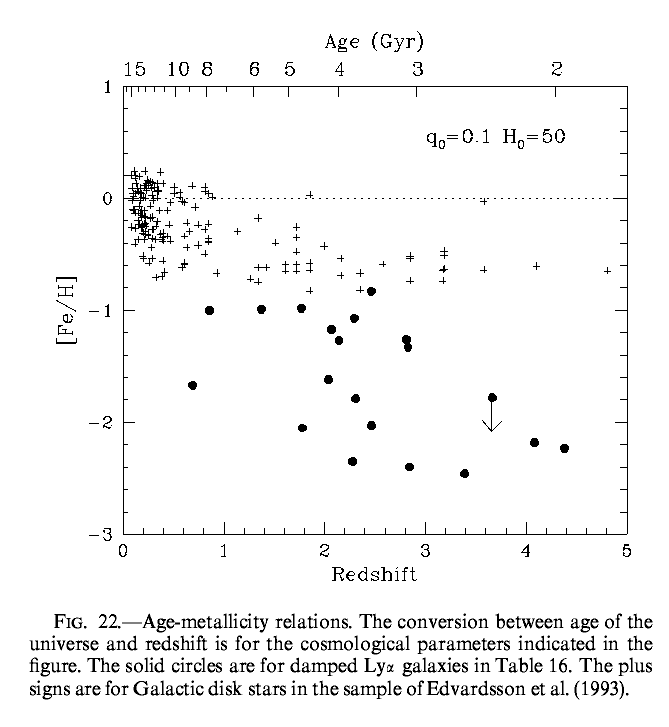
See Abundances at High Redshifts: The Chemical Enrichment History of Damped Ly alpha Galaxies for details about the figure.
So, as time marched on, the ISM became more and more metal-rich.
About five billion years ago, one particular cloud of gas in our own Milky Way began to collapse. This particular cloud was chock full of heavy elements: carbon, oxygen, calcium, iron, even REALLY heavy stuff like lead, platinum, gold and uranium. This cloud became our Solar System.
There is evidence that the proto-Solar nebula was hit by a supernova blast just a very short time before it collapsed; in fact, that blast may have triggered the collapse. The surface of the Earth has been weathered and re-formed and eroded and extruded from volcanos, so terrestrial rocks are unreliable guides to the original composition of the proto-Solar material. But some meteorites appear to have been floating in space for billions of years, virtually unchanged since they formed in the early solar system. Chemical analysis of these pristine meteorites shows that they originally contained radioactive isotopes such as aluminum-26 and calcium-41: unstable isotopes which would have decayed into other varieties in just a few million years. One source for these short-lived isotopes is nearby supernova. However, it's also possible that these isotopes may have formed when high-energy radiation from the young Sun struck ordinary, stable forms of the elements. It's still an open question.
A fraction of the gas and dust in this cloud settled into a disk surrounding the proto-Sun. A tiny bit of the material in this disk clumped together to form the Earth, about four and a half billion years ago. The very young planet was extremely hot, due to radioactive decay and frequent impacts with other bodies. Heavy elements (iron, nickel, etc.) tended to sink to the core of this liquid ball, whereas lighter elements (oxygen, hydrogen, silicon, etc.) rose to the top. Eventually, the crust solidified and cooled to the point that liquid water could cover most of its surface.

Flash forward several billion years and see ... life!
All the atoms in your body have been lying around on or near the surface of the Earth for the past five billion years. They haven't been sitting in a clean room, waiting for you to be born. No, they've been recycled continuously since the planet formed. An oxygen atom in your left earlobe was, quite possibly, at different times,
You're not just "starstuff", as Carl Sagan wrote. You're also "dirtstuff", and worse :-)
For fun, try calculating the probability that the breath you are taking right NOW contains at least one molecule exhaled by George Washington.
About metal-poor stars:
About the possibility that a nearby supernova triggered the collapse of the proto-Solar nebula
 Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.
Copyright © Michael Richmond.
This work is licensed under a Creative Commons License.